Abstract
The slit genes have recently been found to encode proteins with a conserved chemorepulsive activity for axons in invertebrates and vertebrates. We have determined the expression pattern of a slit gene in Xenopus embryos. In the neural tube, slit is expressed at the ventral and dorsal midlines, and the motor neurons. slit is also expressed in a changing pattern in the retina. The full-length Xenopus Slit protein is secreted extracellularly, whereas its receptor Roundabout can not be secreted. Using a myc-tagged secreted Slit protein, we confirmed the binding of Slit to Roundabout expressed on the cell surface.
These results confirm Slit–Roundabout interactions and the biochemical properties of Slit and Roundabout proteins, and further support the idea that Slit may guide axon projections in multiple regions of the embryo.
Keywords: Slit, Roundabout, olfactory bulb, axon guidance, chemorepulsion
Correct projection of axons to their targets is essential for the formation and function of the nervous system. Identification of axon guidance molecules and determination of their expression patterns and functional properties are, therefore, important for our understanding of neural development. Work in the past few years has revealed the function of two families of secreted long-range chemoattractants and chemorepellents, Netrins and Semaphorins (Sema).12,28,38,42,57 Recently, Slit proteins have been found to be a new family of long-range chemorepellents.6,25,33,40
Netrins were identified as attractants existing in the floor plate for axons from commissural interneurons located in the dorsal part of the vertebrate spinal cord.22,49,56 It is functionally conserved in C. elegans and Drosophila.8,16,18,30 Netrins can also be repulsive to specific axons.9,18 There are two types of receptors for netrins: UNC40/DCC/neogenin,7,18,21 and UNC5.1,18,31,32 Sema was identified in chick embryos as an axon collapsing molecule34,35 and in Drosophila as an axon guidance molecule.27 It was soon shown to be an axon repellent.36 The Sema proteins are now known to be a large family with repulsive and attractive activities.2–5,14,41,43,50,51,58,61 Transmembrane receptors for Sema include neuropilins and plexins.10,11,17,20,26,29,54,55,60
The slit gene was initially found in Drosophila to affect larval cuticular patterning.39 slit cDNAs were cloned by Rothberg et al. and shown to encode a large protein with leucine-rich repeats (LRRs) and epidermal growth factor (EGF) repeats.44 slit was thought to play roles in the differentiation of midline glial cells and the separation of longitudinal axonal tracts in the ventral nerve cord of Drosophila embryos.44–46 Vertebrate slit genes have been identified from rats and humans.19,37 Recent studies of Drosophila and vertebrate Slit proteins have shown that they are chemorepellents.6,25,33,40,59 A receptor for Slit is the transmembrane protein Roundabout (Robo),6,25,33 encoded by the robo genes in invertebrates and vertebrates.23,24,48,65 Most recently, Slit has been directly shown to be guide the direction of migrating neurons.63,66
We have isolated cDNAs for slit genes from Xenopus, the chicken and the mouse.33,64 We show here the pattern of slit expression in Xenopus embryos. We also present evidence for extracellular secretion of Xenopus Slit protein. We have carried out experiments to confirm the binding of a Slit protein to Robo, and the chemorepulsive activity of Slit. Results from these studies are complementary to the recent findings of a ligand–receptor relationship between Slit and Robo and a repellent role for Slit.6,25,33,40,59
Experimental Procedures
In situ hybridization
Whole-mount in situ hybridization was performed as described previously with minor modifications.33,62 The color reaction was carried out in 34 μg/ml NBT and 340 μg/ml BCIP. To detect slit mRNA in early embryos such as chick stages 4, 5, 6 and Xenopus stages 12, 13, 14, 15, the color reaction was carried out at 4°C, which increased the signal-to-noise ratio. Sections were obtained after whole-mount in situ hybridization; stained embryos were embedded in 7.5% low-melting-point agarose and 50-μm sections were cut with a Vibratome.
Cell culture and transfection
Human embryonic kidney (HEK) 293 cells or 293T cells were maintained in 10% fetal bovine serum (FBS) in Dulbecco's modified Eagle's medium (DMEM; Gibco). Cells were grown to 70% confluence on 10-cm tissue culture dishes and transfected with approximately 25 μg plasmid DNA per plate using calcium phosphate for 16–24 h. Plasmids expressing a myc-tagged Xenopus Slit and HA-tagged rat Robo-1 were used in transient transfections described in Fig. 4. A stable cell line expressing xSlit-myc and a stable cell line transfected with a control vector were also used in the experiments.
Fig. 4.

Repulsion of olfactory bulb axons by Slit. A–C show different views of the same co-culture with control HEK cells laid on top of the telencephalon. D–F show different views of the same co-culture with Slit expressing cells laid on top of the telencephalon. (A) A bright-field view of the olfactory bulb–telencephalon co-culture. Control cells were laid on top of the telencephalon and DiI was inserted into the olfactory bulb. OB indicates the olfactory bulb and Tel indicates the telencephalon. (B) A fluorescent view of the same co-culture as that shown in A; note that axons from the olfactory bulb projected into the telencephalon. (C) A superimposition of A and B. (D) A bright-field view of the olfactory bulb–telencephalon co-culture. Slit expressing cells were laid on top of the telencephalon. (E) A fluorescent view of the same co-culture as that shown in D; note that axons from the olfactory bulb turned away from the telencephalon. (F) A superimposition of D and E.
Cell surface binding and immunocytochemistry
HEK293 cells grown in 10-cm dishes were transfected with Robo-HA or vector plasmids. Approximately 30 h after transfection, cells were suspended by pipetting up and down several times and then seeded on to 6-well or 24-well dishes to 50% confluence. Cells were grown for another 12–18 h before incubation with Slit-myc. After 1 h of incubation with the conditioned media followed by three to four washes in HBHA buffer (Hanks' balanced salt solution [HBSS], 0.5 mg/ml bovine serum albumin, 20 mM HEPES, pH 7.0), cells were fixed for 30 s in acetone–formaldehyde fixative (60% acetone, 3% formaldehyde, 20 mM HEPES pH 7.0). Cells were then washed three times in HBSS (150 mM NaCl, 20 mM HEPES, pH 7.0). Following three washes, mouse anti-myc and anti-mouse conjugated to Cy3 were used to visualize Slit-myc binding. Green fluorescent protein expression indicated similar transfection efficiencies in vector and Robo-HA transfected cells.
Olfactory bulb axon guidance assay
Collagen gel matrices were prepared according to Guthrie and Lumsden.15 Cell aggregates were prepared according to Fan and Tessier-Lavigne.13 Co-culture of olfactory bulb–telencephalon were carried out according to Sugisaki et al.53 The telencephalic hemisphere and the olfactory bulb were dissected out from E12.5 mice and placed on a collagen gel. Aggregates of cells stably transfected with either vector alone or with slit-myc cDNA were put on top of the telencephalon region, but not on the olfactory bulb. Whole-mount preparations were cultured with DMEM containing 10% FBS at 37°C with 5% CO2. Forty hours later, small crystals of lipophilic dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (DiI; Molecular Probes) were inserted into the olfactory bulbs. Eight hours later, the specimens were fixed with 4% paraformaldehyde in 10 mM phosphate-buffered saline and kept at 4°C before microscopic examination. All experiments were carried in accordance with local and national ethical guidelines on the use and welfare of animals.
Results
Determination of slit expression patterns in Xenopus and chick embryos by in situ hybridization
There are three mammalian homologs of the Drosophila slit gene.6,19,33,37,40,59,64 We have recently isolated cDNAs encoding a full-length Xenopus Slit protein.33 The Xenopus slit is an ortholog of the mouse slit-2 (mslit-2). In the subsequences, an LRR in a Slit from one species is closest to the corresponding LRR in a Slit of another species, whereas the sequence of an EGF repeat in one species is not necessarily closest to the corresponding EGF in another species. The sequence of the chick slit is partial, appears to be closest to the Xenopus sequence.33 Although we have shown the expression pattern of chick slit, the uncertainty in comparing partial sequences made it unclear whether the expression of the Xenopus slit would be similar to that of chick slit. We present here results of in situ hybridization showing the embryonic distribution of Xenopus slit mRNA.
Xenopus slit expression begins at the midline in the late gastrula at stage 12 (Fig. 1A, D), initially in the midline of the dorsal mesoderm including the prechordal plate and the notochord (Fig. 1A, D). slit can be detected in the floor plate and the neural fold in neurula at stage 17 (Fig. 1B, E). After the completion of neural tube closure by stage 19, slit is expressed in the motoneuron columns as well as in the roof plate and the floor plate (see Fig. 1F for a stage 20 embryo). slit is expressed in the eyes of the tailbud after stage 22; the expression pattern in the eyes changes over time: slit is initially expressed in the retina pigment epithelium as well as the ciliary margin (Fig. 1C, H), but later it is expressed in the amacrine cell layer (Fig. 1I). At stage 26, slit expression in the mesodermal midline including the prechordal plate and the notochord begins to disappear, first from the rostral regions and later from caudal regions. Its expression in the floor plate, the roof plate and the motor neurons persists to the latest stage examined (stage 45).
Fig. 1.
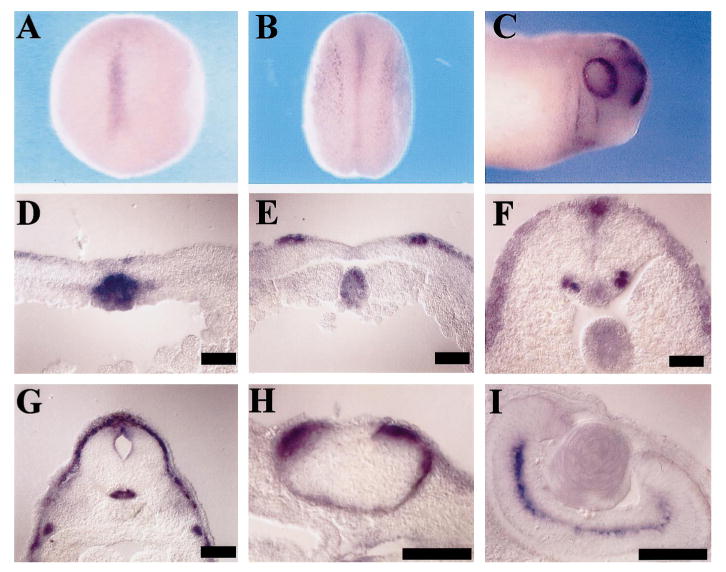
Expression of slit in Xenopus embryos. Results of in situ hybridization are shown here. Scale bars = 100 μm. (A) A dorsal view of a stage 12 embryo showing slit expression at the midline. (B) A dorsal view of a stage 17 embryo showing expression at the midline and the neural fold. (C) A lateral view of a stage 26 embryo showing expression in the retina and branchial clefts. (D) A transverse section of a stage 12 embryo showing expression in the notochord. (E) A transverse section of a stage 17 embryo showing expression in the notochord, and the neural fold. The expression in the floor plate is weak in this section, but is clearly detectable in sections of multiple embryos. (F) A transverse section of a stage 20 embryo showing expression in the notochord, floor plate, the motor neurons, and the roof plate. (G) A transverse section of a stage 35 embryo at the hindbrain level, showing expression in the floor plate and the roof plate. (H) A transverse section of a stage 28 embryo showing expression in the ciliary margin and the retina pigment epithelium layers. (I) A transverse section of a stage 45 embryo showing expression in the amacrine cells in the retina.
Extracellular secretion of Slit, but not Robo, protein
To test whether Slit and Robo proteins can be secreted extracellularly, we expressed Slit and Robo proteins in cultured cells. cDNAs expressing the full-length Xenopus Slit protein tagged with the myc epitope (Slit-myc) and the full-length rat Robo-1 protein tagged with the hemagglutinin (HA) epitope (Robo-HA) were separately transfected into HEK-derived 293T cells. Conditioned media and lysates of cells transfected with Slit-myc or Robo-HA were collected. A monoclonal anti-myc antibody specifically detected and immunoprecipitated the Slit-myc protein in both the culture medium and the lysate of cells transfected with slit-myc cDNA (Fig. 2A). The detection of Slit in the extracellular medium indicated that the full-length Xenopus Slit is a secreted protein. By contrast, Robo-HA could be detected only in the lysate, but not in the conditioned medium of cells transfected with robo-HA (Fig. 2B). This is consistent with the prediction that the full-length Robo protein is a membrane protein.23 Multiple bands lower than the full-length Slit and Robo proteins were observed (Fig. 2A–D), suggesting possible proteolytic cleavage of these proteins.
Fig. 2.
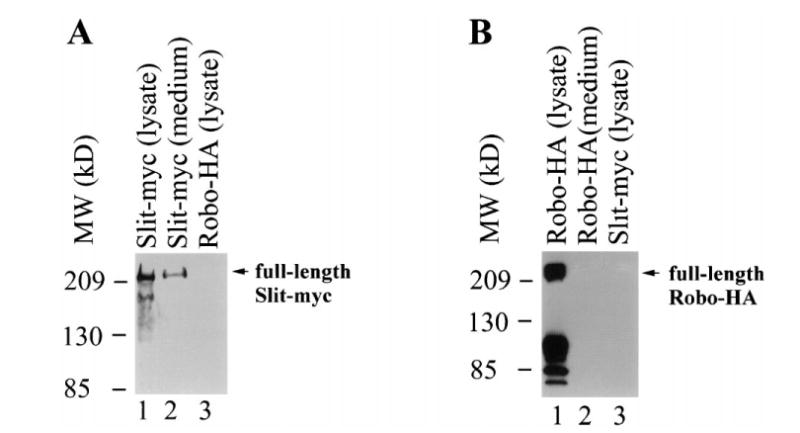
Extracellular secretion of Slit, but not Robo. Shown here are results from western blots. (A) The monoclonal anti-myc antibody detected Slit-myc in both the conditioned medium (lane 1) and lysates (lane 2) of cells transfected with slit-myc, but not the lyate (lane 3) of cells transfected with robo-HA. The upper band is equivalent to the band produced by in vitro translation of an mRNA encoding the full-length Slit-myc. Multiple bands of Slit in addition to the full-length Slit-myc were observed. (B) The monoclonal anti-HA antibody detected Robo-HA in the lysates (lane 1), but not in the medium (lane 2), of cells transfected with robo-HA. It did not recognize Slit-myc (lane 3). The uppermost band is equivalent to the band produced by in vitro translation of an mRNA encoding the full-length Robo-HA. Multiple bands of Robo-HA in addition to the full-length were observed.
Binding of soluble Slit-myc protein to cell surface Robo protein
We have shown that an alkaline phosphatase (AP)-tagged Slit protein (Slit-AP) could bind to Robo expressed on the cell surface.33 Since that fusion protein contained a large AP tag, one question is whether the binding is mediated by Slit part of the fusion protein. We, therefore, tested the ability of Slit-myc, which has only a small myc tag, to bind to the membrane of cells transfected with robo.
The soluble Slit-myc protein was collected from the conditioned medium of cells transfected with slit-myc cDNA and added to the media culturing control cells or cells transfected with robo. After incubation, Slit-myc was detected by the monoclonal anti-myc antibody and a Cy3-conjugated anti-mouse secondary antibody. Slit-myc did not bind to cells transfected with the vector (Fig. 3A, B), whereas its presence was observed after cells were transfected with robo (Fig. 3C, D). Taken together with results from Slit-AP binding to Robo, these results suggest that the binding of Slit fusion proteins to Robo is mediated through the Slit part, not the tags.
Fig. 3.
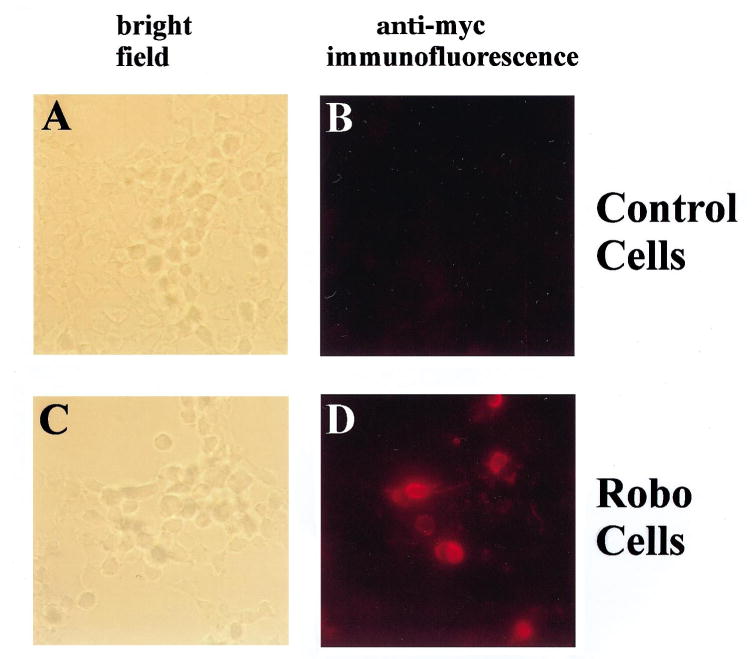
Binding of the soluble Slit-myc protein to robo-transfected cells. Slit-myc was added to cells transfected either with the vector (A, B) or robo-HA (C, D). The anti-myc antibody and Cy3-conjugated anti-mouse secondary antibody were used to detect Slit-myc immunofluorescent staining (B, D). (A) A bright-field view of cells transfected with the vector plasmid. No immunofluorescence was detected after anti-myc staining. (B) The same cells as those shown in A, but viewed under a fluorescence filter. No fluorescent signal was visible to indicate anti-myc staining. (C) A bright-field view of cells transfected with robo-HA. (D) The same cells are shown in C, but viewed under a fluorescence filter. Some of the cells were found to stain for the anti-myc antibody, indicating the binding of Slit-myc to these cells.
Repulsion of olfactory bulb axons by Slit
Using aggregates of cells expressing Slit pre-labeled with the lipophilic dye DiO, we have found that these cells could repel axons from the olfactory bulb.33 To ensure that dye labeling of the cultured cells does not change the properties of the Slit expressing cells, we have repeated these experiments with unlabeled control or Slit expressing cells.
We used the whole-mount co-culture of the olfactory bulb and telencephalon.33,53 During normal development, olfactory bulb axons grow into the telencephalon, forming the lateral olfactory tract.47,52 In the co-culture, two days after the telencephalon region was covered with aggregates of control HEK cells, olfactory bulb axons grew into the telencephalon (n = 24) (Fig. 4A–C). If the telencephalon was covered with Slit expressing HEK cells, olfactory bulb axons could not grow into the telencephalon and instead avoided the telencephalon (n = 12) (Fig. 4D–F). These results confirm that Slit cells can indeed repel olfactory bulb axons projecting to the telencephalon.
Discussion
We have examined the expression pattern of slit in Xenopus embryos, demonstrated the secretion of Slit protein into the extracellular medium, shown the binding of Slit-myc to cells expressing Robo, and confirmed the repulsive activity of the Slit protein.6,25,33,40,59,64
That Slit is a ligand for Robo has been previously suggested by genetic interactions of slit and robo mutants,25 by direct binding of Slit and Robo proteins,6,33,64 and by the binding of Slit-AP to Robo expressed on the cell surface.33 Results presented here show that Slit-myc can also bind to Robo-expressing cells. This rules out the possibility that the large AP tag in the Slit-AP fusion plays a significant role in binding to Robo cells.
The patterns of slit expression in Xenopus and chick embryos are not identical, but quite similar. In chick embryos,33 expression was observed in Hensen's node at stage 4 +. Equivalent expression of Xenopus slit in the Spemann organizer has not been observed. However, most of the other aspects of expression are similar in these two species, including the changing patterns in the notochord and the retina. In both species, the expression patterns suggest that Slit is likely to play roles in multiple regions of the embryo. Slit expression in the ventral midline of the neural tube suggests functions in axon guidance around the midline. For olfactory bulb axons, Slit may drive them to grow laterally on the same side of the midline. For commissural axons in the spinal cord, slit expression in the floor plate may prevent commissural axons that have crossed the floor plate from re-crossing the floor plate. slit expression in the motor neurons may force these commissural axons to turn longitudinally. slit expression in the roof plate suggests that Slit may guide axons around the dorsal midline of the neural tube. slit expression in the retina suggests that Slit functioning is not limited to axon guidance at the midline. Moreover, the changing pattern of slit expression in the retina does not correlate simply with projection of axons from the retina ganglion cells, suggesting possible function in guiding the formation of local circuits within the retina, or functions other than axon guidance.
Acknowledgments
We are grateful to the John Merck Fund, NSFC and SCST for support; to the John Merck Fund, NSFC, and the Leukemia Society of America for scholar awards (to Y. R and J. Y. W.).
Abbreviations
- AP
alkaline phosphatase
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate
- DMEM
Dulbecco's modified Eagle's medium
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- HBSS
Hanks' balanced salt solution
- HEK
human embryonic kidney
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- LRR
leucine-rich repeats
- Robo
Roundabout
- Sema
Semaphorins
References
- 1.Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. doi: 10.1038/386838a0. [DOI] [PubMed] [Google Scholar]
- 2.Adams RH, Betz H, Püschel AW. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev. 1996;57:33–45. doi: 10.1016/0925-4773(96)00525-4. [DOI] [PubMed] [Google Scholar]
- 3.Bagnard D, Lohrum M, Uziel D, Püschel A, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- 4.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 5.Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J molec Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- 6.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Evolutionary conservation of the repulsive axon guidance function of Slit proteins and of their interactions with Robo receptors. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 7.Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 8.Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. A Rev Neurosci. 1995;18:497–529. doi: 10.1146/annurev.ne.18.030195.002433. [DOI] [PubMed] [Google Scholar]
- 9.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant Netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin–neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. doi: 10.1016/s0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 11.Cheng HJ, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 12.Culotti JG, Kolodkin AL. Functions of netrins and semaphorins in axon guidance. Curr Opin Neurobiol. 1996;6:81–88. doi: 10.1016/s0959-4388(96)80012-2. [DOI] [PubMed] [Google Scholar]
- 13.Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a Hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 14.Giger RJ, Wolfer DP, De Wit GMJ, Verhaagen J. Anatomy of rat semaphorin III/collapsin-1 mRNA expression and relationship to developing nerve tracts during neuroembryogenesis. J comp Neurol. 1996;376:378–392. doi: 10.1002/(SICI)1096-9861(19961118)375:3<378::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie S, Lumsden A. Collagen gel coculture of neural tissue. Neuroprotocols. 1994;4:116–120. [Google Scholar]
- 16.Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 18.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 19.Itoh A, Miyabayashi T, Ohno M, Sakano S. Cloning and expressions of three mammalian homologues of Drosophila slit suggests possible roles for Slit in the formation and maintenance of the nervous system. Molec Brain Res. 1998;62:175–186. doi: 10.1016/s0169-328x(98)00224-1. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami A, Kitsukawa T, Takagi S, Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J Neurobiol. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SSY, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 23.Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 24.Kidd T, Russell C, Goodman CS, Tear G. Dosage sensitive and complementary functions of Roundabout and Commissureless control axon crossing of the CNS midline. Neuron. 1998;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- 25.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the Robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 26.Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- 27.Kolodkin AL, Matthes D, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 28.Kolodkin AL. Semaphorins: mediators of repulsive growth cone guidance. Trends Cell Biol. 1996;6:15–22. doi: 10.1016/0962-8924(96)81033-6. [DOI] [PubMed] [Google Scholar]
- 29.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a Semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 30.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. Frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 31.Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–838. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- 32.Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migration in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- 33.Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate Slit, a secreted ligand for the transmembrane protein Roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper J. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron. 1995;14:1131–1140. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 36.Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama M, Nakajima D, Nagase T, Nomura N, Seki N, Ohara O. Identification of high-molecular-weight proteins with multiple EGF-like motifs by motif-trap screening. Genomics. 1998;51:27–34. doi: 10.1006/geno.1998.5341. [DOI] [PubMed] [Google Scholar]
- 38.Nieto MA. Molecular biology of axon guidance. Neuron. 1996;17:1039–1048. doi: 10.1016/s0896-6273(00)80237-8. [DOI] [PubMed] [Google Scholar]
- 39.Nüsslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Roux's Archs dev Biol. 1984;193:267–282. doi: 10.1007/BF00848156. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chédotal A. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 41.Püschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 42.Püschel AW. The semaphorins: a family of axonal guidance molecules? Eur J Neurosci. 1996;8:1317–1321. doi: 10.1111/j.1460-9568.1996.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 43.Püschel AW, Adams RH, Betz H. The sensory innervation of the mouse spinal cord may be patterned by differential expression of and differential responsiveness to semaphorins. Molec cell Neurosci. 1996;7:419–431. doi: 10.1006/mcne.1996.0030. [DOI] [PubMed] [Google Scholar]
- 44.Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S. slit: an EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell. 1988;55:1047–1059. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- 45.Rothberg JM, Jacob JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for the development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- 46.Rothberg JM, Artavanis-Tsakonas S. Modularity of the Slit protein. Characterization of a conserved carboxy-terminal sequence in secreted proteins and a motif implicated in extracellular protein interactions. J molec Biol. 1992;227:367–370. doi: 10.1016/0022-2836(92)90891-m. [DOI] [PubMed] [Google Scholar]
- 47.Schwob JE, Price JL. The development of axonal connections in the central olfactory system of rats. J comp Neurol. 1984;223:177–202. doi: 10.1002/cne.902230204. [DOI] [PubMed] [Google Scholar]
- 48.Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- 49.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd I, Luo Y, Raper JA, Chang S. The distribution of Collapsin-1 mRNA in the developing chick nervous system. Devl Biol. 1996;173:185–199. doi: 10.1006/dbio.1996.0016. [DOI] [PubMed] [Google Scholar]
- 51.Shepherd IT, Luo Y, Lefcort F, Reichardt LF, Raper JA. A sensory axon repellent secreted from ventral spinal cord explants is neutralized by antibodies raised against Collapsin-1. Development. 1997;124:1377–1385. doi: 10.1242/dev.124.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shipley MT, McLean JH, Ennis M. Olfactory system. In: Paxinos G, editor. The Rat Nervous System. Academic; San Diego: 1995. pp. 899–926. [Google Scholar]
- 53.Sugisaki N, Hirata T, Naruse I, Kawakami A, Kitsukawa T, Fujisawa H. Positional cues that are strictly localized in the telencephalon induce preferential growth of mitral cell axons. J Neurobiol. 1996;29:127–137. doi: 10.1002/(SICI)1097-4695(199602)29:2<127::AID-NEU1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 54.Takagi S, Kasuya Y, Shimizu M, Matsuura T, Tsuboi M, Kawakami A, Fujisawa H. Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Devl Biol. 1995;170:207–222. doi: 10.1006/dbio.1995.1208. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi T, Nakamura F, Jin Z, Kalb RG, Strittmatter SM. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nature Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- 56.Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- 57.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 58.Varela-Echavarria A, Tucker A, Püschel AW, Guthrie S. Motor axon subpopulations respond differentially to the chemorepellents netrin-1 and semaphorin D. Neuron. 1997;18:193–207. doi: 10.1016/s0896-6273(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 59.Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Purification of an axon elongation- and branch-promoting activity from brain identifies a mammalian Slit protein as a positive regulator of sensory axon growth. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 60.Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal Semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- 61.Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J comp Neurol. 1995;351:329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- 62.Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD. The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J comp Neurol. 1995;361:321–333. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]
- 63.Wu W, Wong K, Chen JH, Jiang ZH, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the concentration gradient of the secreted protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse Slit family: secreted ligands for Robo expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999 doi: 10.1006/dbio.1999.9371. in press. [DOI] [PubMed] [Google Scholar]
- 65.Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Y, Li HS, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extra-cortical origin to the neocortex. Neuron. 1999;23:31–43. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]


