SUMMARY
Several regulatory concerns have hindered development of androgens as anabolic therapies, despite unequivocal evidence that testosterone supplementation increases muscle mass and strength in men; it induces hypertrophy of type I and II muscle fibers, and increases myonuclear and satellite cell number. Androgens promote differentiation of mesenchymal multipotent cells into the myogenic lineage and inhibit their adipogenic differentiation, by facilitating association of androgen receptors with β-catenin and activating T-cell factor 4. Meta-analyses indicate that testosterone supplementation increases fat-free mass and muscle strength in HIV-positive men with weight loss, glucocorticoid-treated men, and older men with low or low-normal testosterone levels. The effects of testosterone on physical function and outcomes important to patients have not, however, been studied. In older men, increased hematocrit and increased risk of prostate biopsy and detection of prostate events are the most frequent, testosterone-related adverse events. Concerns about long-term risks have restrained enthusiasm for testosterone use as anabolic therapy. Selective androgen-receptor modulators that are preferentially anabolic and that spare the prostate hold promise as anabolic therapies. We need more studies to determine whether testosterone or selective androgen-receptor modulators can induce meaningful improvements in physical function and patient-important outcomes in patients with physical dysfunction associated with chronic illness or aging.
Keywords: anabolic therapies, androgens, sarcopenia, selective androgen receptor modulators, testosterone
INTRODUCTION
The powerful demographic trend towards aging of human populations has focused attention on issues of aging-associated impairments and their socioeconomic impact.1 As men and women grow older, their muscle mass, muscle strength, and leg power decrease because of the loss of skeletal muscle fibers, especially type II fibers. The loss of muscle mass and performance with advancing age might contribute to increased risk of falls, fractures, and disability. Functional decline and dependence in older individuals place a large burden on health-care services.1
Similarly, in many chronic illnesses, such as those associated with HIV infection, chronic obstructive pulmonary disease (COPD), end-stage renal disease, and many types of cancers, we can now achieve disease stability, but not cure. The course of these chronic disorders is punctuated by loss of fat-free mass (FFM)1 and increased risk of disability, dependency, and impaired quality of life. Anabolic therapies, such as androgens, that can restore FFM, muscle strength, and physical function would, therefore, be expected to improve clinical outcomes.
Despite widespread recognition of the anabolic effects of androgens by athletes, the academic community continued to be skeptical because of well-known problems of study design in studies conducted before 1990.2 These problems included the lack of randomization and blinding, the use of relatively small doses of testosterone, and the failure to standardize protein and energy intake and exercise stimulus across treatment groups.
We conducted several meta-analyses in different subject populations to evaluate the anabolic effects of testosterone. These meta-analyses, reported here, provide compelling evidence that testosterone supplementation increases skeletal muscle mass and strength in androgen-deficient and eugonadal young men, older men, and men with many chronic dis orders. The concerns about the long-term risks of testosterone administration have driven pharmaceutical efforts to develop selective androgen receptor modulators (SARMs) that can achieve the anabolic effects without the adverse effects associated with testosterone. Ironically, in spite of the growing evidence of the anabolic effects of androgens (in both healthy men and in men with chronic disorders) and in spite of the substantial pharmaceutical effort to develop SARMs with anabolic activity, no new androgenic compound has yet been approved for any anabolic indication. The slow pace of clinical development of these agents as anabolic therapies illustrates the regulatory and conceptual barriers that have hindered this field.
EVIDENCE OF ANABOLIC EFFECTS OF TESTOSTERONE IN HUMANS
Androgen-deficient men have lower FFM and higher fat mass than do eugonadal controls.3 Levels of bioavailable testosterone correlate with appendicular skeletal muscle mass and lower-extremity strength in older men.4,5 An inverse correlation between testosterone levels and visceral fat mass has also been reported, although it is unclear whether this relationship is independent of changes in sex-hormone-binding globulin levels.6 Suppression of testosterone levels in healthy men is associated with a reduction in FFM and fractional muscle protein synthesis, and an increase in fat mass.7
To evaluate the evidence of the anabolic effects of testosterone, we conducted meta-analyses of testosterone trials in five populations: healthy, hypogonadal men; HIV-infected men with weight loss; HIV-infected women with weight loss; glucocorticoid-treated men; and middle-aged and older men. In our systematic review of testosterone trials in hypogonadal men, we searched MEDLINE and PubMed from 1965 until 2005 for original, full-text, English-language articles focusing on trials that used testosterone or its esters in replacement doses. The search terms used were “hypogonadal men”, “testosterone” and “body composition”.8-17 We also searched the references of identified articles for further papers. Testosterone replacement in hypogonadal men was associated with an average 1.7 kg gain in FFM (95% CI 1.52–1.96) and 1.1 kg gain in body mass. Some studies reported improvements in maximal voluntary strength8,11,12 and a decrease in fat mass (−0.7 kg, 95% CI −1.0 to −0.5).11,13,14,16
In eugonadal men, administration of supraphysiologic doses of testosterone increases FFM, muscle size, and maximal voluntary strength.18 resistance exercise training and recombinant human growth hormone augment the anabolic response to androgens.18,19 The gains in FFM, muscle size, and muscle strength in response to testosterone administration correlate with increasing testosterone dose and circulating testosterone concentrations.20-22 There is consider able heterogeneity in testosterone levels and the anabolic response during testosterone administration. The factors that contribute to variation in anabolic response to androgen administration are poorly understood; polymorphisms in the androgen receptor (AR) gene account for only a small fraction of this variance.22
GLOSSARY
- RESISTANCE EXERCISE TRAINING
Exercise training in which the skeletal muscle moves against resistance; designed to increase muscle mass and strength
The effects of testosterone on muscle performance are domain-specific; it increases maximal voluntary strength and leg power, but does not affect fatigability or specific muscle tension.23 Muscle-strength gains during testosterone administration are proportional to the increase in muscle mass;23 unlike resistance exercise training, testosterone does not improve the contractile properties of skeletal muscle. Androgens do not affect endurance measures, such as maximum rate of oxygen consumption or lactate threshold.
MECHANISMS OF ANABOLIC EFFECTS OF TESTOSTERONE ON MUSCLE
Testosterone-induced increase in muscle mass is associated with hypertrophy of both type I and type II fibers24 and an increase in the number of myonuclei and satellite cells.25 Testosterone promotes the differentiation of mesenchymal, multipotent cells into the myogenic lineage and inhibits their differentiation into the adipogenic lineage.26 Androgens regulate mesenchymal multipotent cell differentiation by binding to ARs, and promoting the association of ARs with β-catenin and translocation of the AR–β-catenin complex into the nucleus, resulting in activation of T-cell-specific transcription factor 4 (TCF-4).27 The activation of TCF-4 modulates a number of wnt-regulated genes that promote myogenic differentiation and inhibit adipogenic differentiation.
- MYONUCLEI
The nuclei inside the muscle fiber cells that are distinguished from satellite cells by their location inside the sarcolemma
- SATELLITE CELLS
Mesenchymal precursor cells that are located inside the basilar lamina but outside the sarcolemma, and are important in muscle fiber hypertrophy and regeneration
- WNT-REGULATED GENES
The WNT signaling pathway is important in the regulation of adipogenic and myogenic differentiation, achieved by modulating the expression of genes, referred to as WNT-regulated genes
Testosterone also inhibits preadipocyte differentia tion into adipocytes.27,28 The effects of testosterone on myogenic differentiation in vitro are blocked by the AR antagonist bicalutamide, indicating that these effects are mediated through an AR pathway.27 It is possible that androgens might exert additional effects through nongenomic mechanisms. Testosterone increases fractional muscle protein synthesis and improves the reutilization of amino acids by muscle.7
We do not know whether conversion of testosterone to dihydrotestosterone (DHT) is required for mediating androgen effects on muscle. Steroid 5-α reductase 2, the product of SRD5A2, which converts testosterone to DHT, is expressed at low concentrations in muscle,29 but individuals with congenital SRD5A2 deficiency have normal muscle development at puberty.
Humans and mice that are null for the cyto-chrome-P450-linked CYP19 aromatase gene30,31 have higher fat mass and lower muscle mass than their wild-type counterparts. Thus, aromatization of testosterone might be important in mediating androgen effects on body composition.
THE ANABOLIC EFFECTS OF TESTOSTERONE IN CHRONIC ILLNESS
Cross-sectional surveys32-34 report a high prevalence of low testosterone levels in HIV-infected men, even in those receiving highly active anti-retroviral therapy. Low testosterone levels, the consequence of multifactorial defects at hypothalamic–pituitary as well as at testicular levels, are associated with weight loss,35 loss of muscle mass and exercise capacity,36 wasting, and progression to AIDS.37
We searched for English-language, full-length original articles in MEDLINE and PubMed from inception to June 2005 for randomized, placebo-controlled trials of longer than 12 weeks duration that studied androgen therapy in HIV-infected men, older than18 years of age. The search terms used were “androgen”, HIV”, “men” and “weight loss”. We searched the reference lists of identified articles for additional papers. Three reviewers selected eight trials38-45 that reported body-weight change and/or body composition in 423 participants. The trials were variable in quality, and hetero geneous in terms of inclusion criteria, treatment dose and duration, blinding, and methods of outcome measurements. We pooled these trials and analyzed the data using a random effects model. Significant inconsistency was found across trials, such that 57% of the between-study differences in body-mass contrasts, and 73% in lean-body-mass (LBM) contrasts, cannot be explained by chance. Androgen supplementation of 3–6 months duration was associated with greater gains than placebo in FFM (contrast 1.4 kg, 95% CI 0.7–2.1), LBM (contrast 1.3 kg, 95% CI 0.4–2.2), and body weight (contrast 1.1 kg, 95% CI 0.2–2.0; Figure 1A, B). In two38,42 of three trials that measured muscle strength, testosterone administration was associated with significantly greater improvements in muscle strength than placebo. Studies using testosterone esters administered a higher dose and reported greater increments in FFM than those using transdermal preparations.46
Figure 1.
Changes in body composition in testosterone-treated HIV-infected men and women. Meta-analysis plots of contrasts between androgen-treated and placebo-treated HIV-infected men with weight loss, showing the change in whole body mass (A), and lean body mass (B) and HIV-infected women with weight loss, showing the change in whole body mass (C) and fat-free mass (D). A positive difference is a favorable testosterone effect on body mass, lean body mass, and fat-free mass. Some studies reported fat-free mass and some lean body mass. Fat-free mass equals lean body mass plus bone mineral content. Confidence intervals that do not overlap with zero represent significant differences between placebo and testosterone groups.
In four trials that reported effects on depression in HIV-infected men,47-50 testosterone therapy had a modest effect on depression indices (−0.6, 95% CI −1.0 to −0.2). Testosterone administration did not improve quality of life.
Changes in CD4+ T lymphocyte counts, HIV copy number, prostate-specific antigen (PSA), HDL cholesterol and adverse event rates were not significantly different between placebo and testosterone groups.38,39,41,42,46 Our systematic review agrees with a previous analysis46 in demonstrating that testosterone supplementation of HIV-infected men with low testosterone levels and weight loss induces modest gains in body weight and FFM, and improved mood. This inference is weakened by in consistent results, heterogeneity in eligibility criteria, disease status, testosterone formulations and doses, treatment duration, and methods of body-composition analysis. There are no data on the effects of testosterone on physical function and disability, or on long-term safety.
Testosterone supplementation of HIV-infected women in doses that raised testosterone levels into the high-normal range for women was safe, but ineffective in promoting significant gains in body weight (+0.3 kg, 95% CI −0.7 to +1.3) or FFM (0.5 kg, 95% CI −0.1 to +1.2; Figure 1C, D).51-53 No significant difference in muscle strength change between placebo-treated and testosterone-treated women was noted in one study;53 another study52 reported greater gains in muscle strength in testosterone-treated women than placebo-treated women, but the magnitude of change was not clinically significant. Testosterone doses used in trials of HIV-infected women51-53 were considerably smaller than those used in HIV-infected men.38-45
OTHER POTENTIAL INDICATIONS FOR USING TESTOSTERONE AS AN ANABOLIC THERAPY
Glucocorticoid administration in pharmacologic doses is associated with muscle atrophy and a high frequency of low testosterone levels owing to suppression at all levels of the hypo thalamic–pituitary–testicular axis.54 In two randomized, placebo-controlled trials,55,56 testosterone supplementa tion of men receiving glucocorticoids for bronchial asthma or COPD was associated with an average 2.3 kg (95% CI 2.0–3.6) greater gain in LBM and a 3.1 kg greater decrease in fat mass (95% CI −3.5 to −2.8) than placebo. These trials found an increase of 4% in bone mineral density in the lumbar spine (95% CI 2–7%); the effect on femoral bone density was not significant.55,56 There are no data on the effects of testosterone supplementation on bone fractures. These inferences are weakened by small sample size and high rates of loss to follow-up.55
Muscle wasting and dysfunction are correctable causes of exercise intolerance in patients with COPD. In one study,57 testosterone replacement increased FFM, muscle size and strength in men with COPD who had low testosterone levels; in another study,58 a low dose of nandrolone induced modest increases in FFM and respiratory muscle strength. Androgens are also being considered for the treatment of physical dysfunction associated with end-stage renal disease, many types of cancer, postoperative state (especially after hip and knee surgery), burn injuries, congestive heart failure, and the re cuperative stage of acute debilitating illnesses.
ANABOLIC EFFECTS OF TESTOSTERONE REPLACEMENT IN OLDER MEN
We searched for original English-language, full-length articles in MEDLINE and PubMed from inception until June 2005 focusing on randomized, controlled trials of longer than 90 days duration reporting the effects on body composition of testosterone or its esters in replacement doses in men aged older than 45 years with low or low-normal testosterone levels. Search terms used were “testosterone”, “older men”, “body composition” and “randomized trials”. One additional study59 was added during expert scrutiny of publications. Three independent observers selected seven trials9,19,60-66 for inclusion. One trial that recruited both young and older men was included as a majority of participants in this study were older than 45 years of age9 and it met all other inclusion criteria. The reports were variable in quality, and heterogeneous in terms of inclusion criteria, testosterone dose and duration of treatment, blinding, and outcome measurements. We pooled these trials (Figure 2) using a random-effects model; for body mass and hand grip, the trials yielded consistent answers, but there was significant inconsistency for fat mass and LBM outcomes (with 88% and 77% of between-study differences not explained by chance). In seven such trials,9,19,60-66 testosterone replacement was associated with a significantly greater increase in LBM (contrast 2.7 kg, 95% CI 1.6–3.7) and grip strength (contrast 3.3 kg, 95% CI 0.7–5.8), and a greater reduction in fat mass (contrast −2.0 kg, 95% CI −3.1 to −0.8) than placebo. The body-weight change did not differ significantly between groups (contrast −0.6 kg, 95% CI −2.0 to +0.8).
Figure 2.
Changes in body composition in testosterone-treated older men. Meta-analysis plots of contrasts between testosterone-treated and placebo-treated men in the change (in kilograms) in whole body mass (A), lean body mass change (B), right-hand grip strength (C), and whole body fat mass (D) in middle-aged and older men more than 45 years of age. A positive difference is a favorable testosterone effect on body mass, lean body mass, and fat-free mass, and a negative difference is a favorable testosterone effect on fat mass. Confidence intervals that do not overlap with zero represent significant differences between placebo and testosterone groups.
Changes in lower-extremity muscle strength and measures of physical function were reported only in a few studies and were in consistent. One study reported no changes in physical function,59 whereas another reported an improvement in physical function.61 As noted by the Institute of Medicine Expert Panel on the Future of Testosterone Research,67 these studies did not have sufficient power to detect changes in clinical outcomes or disability. The studies were performed in asymptomatic men; the effects of testosterone in older men with physical dysfunction are unknown.
ADVERSE EFFECTS OF TESTOSTERONE IN MIDDLE-AGED AND OLDER MEN
The potential side effects of testosterone include acne, oiliness of skin, increased growth of body hair, breast tenderness and enlargement, balding, increase in hemoglobin, sleep apnea, leg edema, and weight gain.66,68 Serious side effects such as hepatotoxicity and hepatic neoplasms have not been observed when testosterone or its esters are administered parenterally or transdermally.
We searched for original articles in MEDLINE and PubMed from inception to June 2005 focusing on randomized trials of longer than 90 days duration, in men older than 45 years with low or low-normal testosterone, to determine the risks of adverse events associated with testosterone replacement (Table 1).69 The search terms used were “testosterone”, “human”, “middle-aged and older men” and “randomized trial”. In 19 studies that met our eligibility criteria, the combined rate of all prostate events was significantly greater in testosterone-treated men than in placebo-treated men (pooled odds ratio 1.78, 95% CI 1.07–2.95). Rates of prostate cancer, serum PSA above 4 ng/ml, and prostate biopsies were numerically higher in the testosterone group than in the placebo group, although differences between groups were not individually statistically significant. As prostate biopsies are often performed in response to an increase in PSA levels, testosterone-treated men were more likely to undergo prostate biopsy than placebo-treated men. Thus, there is considerable bias towards greater number of prostate biopsies and detection of greater number of prostate events in testosterone-treated men than in placebo-treated men; future trials should in corporate strategies to minimize this bias.
Table 1.
Pooled odds ratios for adverse events in randomized, clinical trials of testosterone in older men.
| Event | Adverse event rate for testosterone |
Adverse event rate for placebo |
Pooled odds ratio (95% CI) |
|---|---|---|---|
| Prostate biopsies | 38.7 | 2.8 | 1.87 (0.84–4.15) |
| Prostate cancers | 9.2 | 8.3 | 1.09 (0.48–2.49) |
| PSA >4 ng/ml or 1.5 ng/ml increase during study |
57.1 | 41.6 | 1.19 (0.67–2.09) |
| Increase in IPSS score | 5.5 | 2.8 | 1.08 (0.46–2.52) |
| Acute urinary retention | 2.2 | 0 | 0.99 (0.40–2.44) |
| All prostate events | 112.4 | 55.7 | 1.78 (1.07–2.95)a |
| Hematocrit >50% | 64.5 | 2.8 | 3.69 (1.82–7.51)a |
| Atrial fibrillation or arrhythmia | 9.2 | 2.8 | 1.22 (0.53–2.81) |
| Myocardial infarction | 7.4 | 8.3 | 0.99 (0.44–2.26) |
| Chest pain or ischemia | 7.4 | 8.3 | 0.93 (0.39–2.26) |
| Coronary procedure or CABG | 3.7 | 13.9 | 0.79 (0.35–1.79) |
| Vascular events or cerebrovascular accidents |
5.5 | 11.1 | 0.86 (0.38–1.95) |
| All cardiovascular events | 33.2 | 44.3 | 1.14 (0.59–2.20) |
| Death | 0 | 5.5 | 0.78 (0.32–1.93) |
The adverse event rate (shown per 1,000 patient-years) was calculated on the basis of an average study duration of ten months, standardized to one year and multiplied by 1,000 for testosterone-treated or placebo-treated men. Adapted from Calof et al.69 Copyright © The Gerontological Society of America. Reproduced by permission of the publisher.
Here, the incidence of adverse events was significantly higher in the testosterone group.
CABG, coronary artery bypass graft; IPSS, international prostate symptom score; PSA, prostate-specific antigen.
Testosterone-treated men were nearly four times as likely to experience a hematocrit greater than 50% compared with placebo-treated men (Table 1). Hematocrit increase, the most frequent adverse event associated with testosterone administration, was dose-related.21 Older men demonstrated greater increments in hematocrit than younger men.21 The frequency of cardiovascular events, sleep apnea or death was not significantly different between groups. These data reaffirm the need to monitor hematocrit, PSA and perform regular prostate examinations during testosterone replacement of older men. The long-term effects of testosterone supplementation on prostate and cardiovascular outcomes are unknown.62
There is a trade-off between the testosterone dose and its anabolic and adverse effects. The higher doses induce greater anabolic effects, but are associated with higher frequency of adverse effects.21 The recognition that the magnitude of the anabolic effects that can be achieved with testosterone is inherently limited by its adverse effects has provided the impetus for the develop ment of SARMs.
DEVELOPMENT OF SELECTIVE ANDROGEN RECEPTOR MODULATORS
Concerns about long-term risks of prostate and cardiovascular disorders in older men treated with testosterone have encouraged considerable investment in the development of SARMs that have anabolic effects on the muscle, but do not have adverse effects on prostate and cardiovascular outcomes.68 Nonsteroidal SARMs differ from testosterone and steroidal androgens in several respects. Unlike testosterone, which is converted to active metabolites (i.e. estradiol and DHT), nonsteroidal SARMs do not undergo aromatization or 5-α-reduction, and act as agonists in muscle and bone and as partial agonists in prostate and seminal vesicles. Some nonsteroidal SARMs have more favorable pharmacokinetics, greater AR specificity, and are more amenable to structural modifications, than their steroidal counterparts.
The differing interactions of steroidal and nonsteroidal compounds with ARs could contribute to their unique pharmacologic actions (Figure 3). Bicalutamide, an androgen-receptor antagonist, adopts a greatly bent conformation (Figure 3B) in the AR. Although the A-ring and amide bond of the bicalutamide molecule overlap the steroidal plane (Figure 3C, D), the B-ring folds away from the plane, pointing to the top of the ligand-binding pocket, forming a unique structural feature of this ligand class. The A-ring cyano group forms H-bonds with Q711 and R752, similar to 3-keto group in 5α-DHT (Figure 3A). The chiral hydroxyl group forms H-bonds with L704 and N705, mimicking ring-C and the 17β-OH group in 5α-DHT. These H-bonding interactions are critical for high binding affinity.
Figure 3.
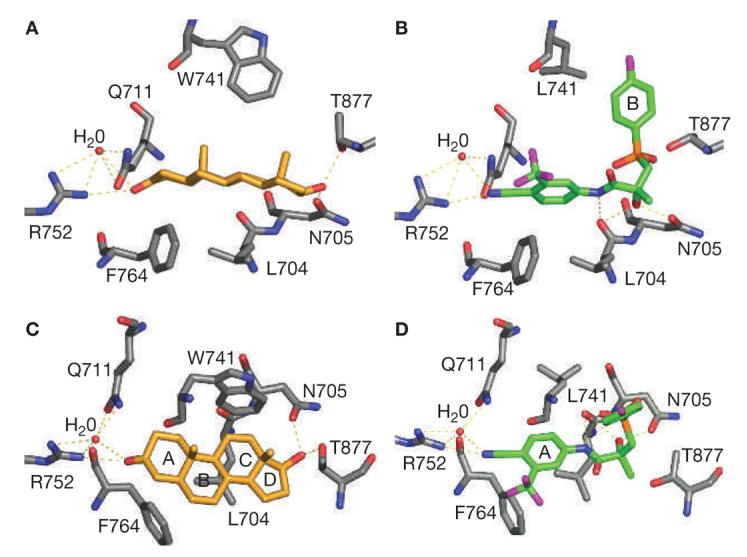
Steroidal (dihydrotestosterone) and nonsteroidal (R-bicalutamide) ligand interactions with the androgen receptor ligand-binding domain binding-pocket. R-bicalutamide (green) W741L complex and dihydrotestosterone (gold) wild-type complex, shown as side views (A and B) and top views (C and D) of the steroidal plane. Ligand and receptor interact mainly through H-bonds (labeled as yellow dotted lines) and hydrophobic interactions. The A-ring and amide bond of the bicalutamide molecule overlaps the steroidal plane of dihydrotestosterone (rings A–D) and shares similar H-bonding pattern, whereas the B-ring (B) folds away from the plane, pointing to the top of the ligand-binding pocket and forming the unique structural feature of this ligand class.
SARM pharmacophores can be classified into four categories (Figure 4): aryl propionamide, bicyclic hydantoin, quinoline, and tetrahydroquinoline analogs. Structural modifications of aryl propionamide analogues bicalutamide and hydroxyflutamide led to the discovery of the first-generation SARMs. These nonsteroidal SARMs are orally available,70 metabolized mostly by amide-bond hydrolysis and A-ring nitro reduction and eliminated exclusively through hepatic metabolism.71
Figure 4.
Four general classes of selective androgen receptor modulator pharmacophores. Information about the stage of development was obtained through company websites.
Nonsteroidal compounds S1 and S4 bind AR with high affinity (Figure 5), and demonstrate tissue selectivity in animal models.72 In castrated rats, both ligands prevented castration-induced tissue atrophy, behaving as partial agonists in the prostate (median effective dose [ED50] = 2 mg/kg/day) but full agonists in levator ani muscle (ED50 = 0.6 mg/kg/day). Prolonged treatment (8 weeks) with S4 restored tissue weight 3 months after castration. At a dose of 3 mg/kg/day, S4 partially restored prostate weight to less than 20% of that in intact rats but fully restored levator ani weight; S4 also restored skeletal muscle strength, bone mineral density and bone strength, LBM, and suppressed luteinizing hormone and follicle-stimulating hormone production.73,74 Furthermore, S4 prevented gonadectomy-induced bone loss in a female rat model of osteoporosis.73 The ability of SARMs to promote both muscle strength and bone mechanical strength constitutes a unique advantage over other therapies for osteoporosis that only increase bone density.73
Figure 5.
Structures and relative binding affinities of some selective androgen receptor modulators. DHT, dihydrotestosterone; RBA, relative binding affinity compared with synthetic ligand R1881; THQ, tetrahydroquinolone.
GLOSSARY
- LEVATOR ANI
A muscle in the pelvic floor that is highly responsive to changes in androgen concentrations and has been used widely as an assay for the anabolic activity of androgenic steroids
In intact male rats, S1 and S4 act as antagonists in the prostate, but as full agonists in levator ani; such SARMs with antagonistic or low intrinsic activity in prostate might be useful in the treatment of benign prostatic hyperplasia or prostate cancer. The suppressive effects of selected members of this class of SARMs on gonadotropin secretion in rats74 suggest a potential application for male contraception.
Ether linkage and B-ring para-position substitution are critical for agonist activity of aryl propionamide SARMs (e.g. S1 and S4 in Figure 5).72 Compounds with ether linkage appear to adopt a more compact conformation than bicalutamide, because of formation of an intramolecular H-bond, allowing the B-ring to avoid steric conflict with the side-chain of W741 in wild-type AR, potentially explaining the agonist activity.75
Hydantoin derivatives are novel tissue-selective SARMS that have an A-ring structure similar to bicalutamide (Figure 5).74 The cyano or nitro group is thought to interact with Q711 and R752.74 The benzene ring or the naphthyl group, together with the hydantoin ring, overlaps the steroid plane, whereas the hydantoin ring nitrogen forms an H-bond with N705. BMS564929 binds AR with high affinity and specificity, as the hydantoin nitrogen or hydroxyl group forms a favorable interaction with T877. Related compounds incorporating a 2,2,2 ring system act as AR antagonists, suggesting that additional steric bulk is unfavorable.
GLOSSARY
- STERIC BULK
Steric bulk refers to the manner in which the bulk of a molecule or group hinders or prevents interaction of other molecules or groups with a specific site in the receptor molecule
In castrated rats, BMS564929 demonstrated tissue selectivity and potent suppression of luteinizing hormone. BMS564929 is orally available in humans, with an in vivo half-life of 8–14 h. The prolonged half-life of these ligands in rats could explain the lower dose needed to achieve pharmacologic effects; differences in in vivo activities of SARMs that share similar binding affinity and intrinsic activity could be related to tissue exposure of these ligands.
Hanada et al.76 reported results of a series of tetrahydroquinoline derivatives as tissue-selective AR agonists for bone. Although these compounds (Figure 5) displayed high AR affinity and strong agonist activity in the prostate and levator ani, they demonstrated little tissue selectivity between androgenic and anabolic tissues. Also, significant in vivo activity was observed only at high sub cutaneous doses, which could be related to their pharmacokinetic profiles.
POTENTIAL MECHANISMS OF TISSUE SELECTIVITY OF SELECTIVE ANDROGEN RECEPTOR MODULATORS
Several mechanisms77,78 have been proposed to explain the tissue selectivity of nuclear receptor modulators. Ligand binding induces specific conformational changes in the ligand-binding domain (LBD), which could modulate surface topology and subsequent protein–protein interactions between AR and other coregulators. Differences in ligand-specific receptor conformation and protein–protein interactions could result in tissue-specific gene regulation, because of unique interactions with androgen response elements, coregulators or transcription factors.
Ligand-induced protein–protein interactions contribute to interactions between amino-terminal and carboxy-terminal ends of the AR (i.e. N–C interaction)79 and coactivator recruitment with the AR. Both interactions are mediated by the interaction between the activator function 2 (AF2) region of AR and ‘FxxLF’ or ‘LxxLL’ binding motifs.80,81 The hydrophobic groove present in AF2 region of AR LBD favors phenylalanine binding, which suggests that N–C interaction is preferred. Although AR–LBD conformation after SARM binding has not been well characterized, some steroidal SARMs that have agonist activity in vitro induce an ‘activating’ conformational change without facilitating N–C interactions,82 suggesting that ligand-specific conformational change is achievable with synthetic ligands.
The tissue selectivity of SARMs could be related to differences in their tissue distribution, potential interactions with steroid 5 α-reductase 2 or CYP19 aromatase, or tissue-specific expression of coregulators.83 Autoradiography studies with bicalutamide and hydantoin derivatives showed that these drugs do not accumulate preferentially in skeletal muscle. Testosterone actions in some androgenic tissues are amplified by conversion to 5α-DHT; however, nonsteroidal SARMs do not serve as substrates for SRD5A2. Tissue selectivity has also been demonstrated in testosterone-treated intact rats in the presence of SRD5A2 inhibitors.
Slight structural modifications can change the ligand from an antagonist to an agonist. Favorable H-bonding between ligand and the T877 side chain, a feature that mimics the 3-keto group of testosterone, and hydrophobic interactions are critical for high affinity ligand-binding and stimulation of AR action. It remains unclear how ligand–receptor interaction determines agonist or antagonist activity of the ligand because of the limited knowledge of receptor conformation upon ligand binding. The mechanisms that confer tissue selectivity are poorly understood. SARMs with various intrinsic activities provide an excellent opportunity to understand the role of AR–protein and/or AR–DNA interaction in tissue selectivity of SARMs.
CONCLUSIONS
Androgens increase muscle mass and some aspects of muscle performance; however, a number of regulatory and conceptual issues have hindered clinical applications of these anabolic effects. A lack of consensus on how to define the concept of symptomatic physical impairment has made it difficult to develop criteria for subject selection. The ongoing debate over clinical conditions for which approval of these anabolic therapies should be sought is not fully resolved. This issue is crucial for seeking regulatory approval and, consequently, for financial reimbursement for these anabolic therapies.
Experts disagree on what outcomes should constitute evidence of efficacy in clinical trials of testosterone and SARMs. Demonstration of significant increments in muscle mass and muscle strength in clinical trials of androgens is necessary but insufficient to prove efficacy. In addition to improvements in muscle mass and strength, it would be necessary to demonstrate gains in physical function and health-related outcomes. There is uncertainty about which measures of physical function are androgen-responsive, and whether androgen administration can improve measures of functional impairment and disability. We do not know what magnitude of change in physical function is important to patients. It is, therefore, not clear what assumptions concerning the minimum important difference should be used to guide sample size estimates. Long-term studies to resolve persistent concerns about prostate and cardiovascular safety would require large samples and pose significant fiscal and logistic challenges. SARMs that are preferentially anabolic and are free from the adverse effects of testosterone have considerable appeal as anabolic therapies. Nonsteroidal SARMs do not, however, undergo 5-α reduction or aromatization; therefore, their safety profile might differ considerably from that of testosterone. The enormous promise of testosterone and SARMs as anabolic therapies would be realized only through the implementation of bold and imaginative efficacy trials that overcome these conceptual hurdles.
KEY POINTS.
Meta-analyses of clinical trials provide unequivocal evidence that testosterone administration increases skeletal muscle mass, muscle strength, and leg power; these anabolic effects of testosterone are dose-related
The effects of testosterone supplementation on physical function and clinical outcomes in older men with physical dysfunction and in men with chronic illness are unknown
Testosterone induces skeletal muscle fiber hypertrophy and increases the number of satellite cells
Testosterone increases muscle mass and decreases fat mass by promoting the differentiation of mesenchymal multipotent cells into myogenic lineage and inhibiting their differentiation into the adipogenic lineage
An increase in hematocrit and increased risk of detection of prostate events are the most frequent adverse effects of testosterone administration in older men; the anabolic applications of testosterone are constrained by dose-limiting adverse events and concerns about long-term effects on the prostate and cardiovascular risk
Nonsteroidal selective androgen-receptor modulators that are free of adverse effects of testosterone and are preferentially anabolic hold great promise as anabolic therapies
Footnotes
REVIEW CRITERIA
We searched MEDLINE and PubMed from 1965 until June 2005 for original, full-text, English-language articles. Search terms were “hypogonadal men”, “testosterone”, “body composition”, “androgen”, “HIV”, “men”, “weight loss”, “older men”, “randomized trials” and “middle-aged and older men”. We also searched references of identified articles.
Competing interests
SB, OMC, TWS, NAM and JTD declared competing interests; go to the article online for details. The other authors declared they have no competing interests.
References
- 1.Spillman BC, Lubitz J. The effect of longevity on spending for acute and long-term care. N Engl J Med. 2000;342:1409–1415. doi: 10.1056/NEJM200005113421906. [DOI] [PubMed] [Google Scholar]
- 2.Bhasin S, et al. Androgen effects on body composition. Growth Horm IGF Res. 2003;13(Suppl A):S63–S71. doi: 10.1016/s1096-6374(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 3.Katznelson L, et al. Using quantitative CT to assess adipose distribution in adult men with acquired hypogonadism. AJR Am J Roentgenol. 1998;170:423–427. doi: 10.2214/ajr.170.2.9456958. [DOI] [PubMed] [Google Scholar]
- 4.Roy TA, et al. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283:E284–E294. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 6.Seidell JC, et al. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 7.Mauras N, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 9.Steidle C, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 10.McNicholas TA, et al. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int. 2003;91:69–74. doi: 10.1046/j.1464-410x.2003.04016.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, et al. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3654–3662. doi: 10.1210/jcem.81.10.8855818. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Endocrinol Metab. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 13.Katznelson L, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 14.Brodsky IG, et al. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 15.Dobs AS, et al. Interrelationships among lipoprotein levels, sex hormones, anthropometric parameters, and age in hypogonadal men treated for 1 year with a permeation-enhanced testosterone transdermal system. J Clin Endocrinol Metab. 2001;86:1026–1033. doi: 10.1210/jcem.86.3.7285. [DOI] [PubMed] [Google Scholar]
- 16.Snyder PJ, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin S, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 19.Blackman MR, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 20.Bhasin S, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 21.Bhasin S, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 22.Woodhouse LJ, et al. Development of models to predict anabolic response to testosterone administration in healthy young men. Am J Physiol Endocrinol Metab. 2003;284:E1009–E1017. doi: 10.1152/ajpendo.00536.2002. [DOI] [PubMed] [Google Scholar]
- 23.Storer TW, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 24.Sinha-Hikim I, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 25.Sinha-Hikim I, et al. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–E205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- 26.Singh R, et al. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 27.Bhasin S, et al. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci. 2003;58:M1103–M1110. doi: 10.1093/gerona/58.12.m1103. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with β-catenin and TCF4 may bypass canonical Wnt signaling to downregulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartsch W, et al. Regulation and compartmentalization of androgens in rat prostate and muscle. J Steroid Biochem. 1983;19:929–937. doi: 10.1016/0022-4731(83)90036-5. [DOI] [PubMed] [Google Scholar]
- 30.Carani C, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 31.Jones ME, et al. Aromatase deficient (ArKO) mice have phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rietschel P, et al. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clin Infect Dis. 2000;31:1240–1244. doi: 10.1086/317457. [DOI] [PubMed] [Google Scholar]
- 33.Dobs AS, et al. Serum hormones in men with human immunodeficiency virus-associated wasting. J Clin Endocrinol Metab. 1996;81:4108–4112. doi: 10.1210/jcem.81.11.8923868. [DOI] [PubMed] [Google Scholar]
- 34.Arver S, et al. Serum dihydrotestosterone and testosterone concentrations in human immunodeficiency virus-infected men with and without weight loss. J Androl. 1999;20:611–618. [PubMed] [Google Scholar]
- 35.Coodley GO, et al. Endocrine function in the HIV wasting syndrome. J Acquir Immune Defic Syndr. 1994;7:46–51. [PubMed] [Google Scholar]
- 36.Grinspoon S, et al. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab. 1996;81:4051–4058. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- 37.Salehian B, et al. Testicular pathologic changes and the pituitary–testicular axis during human immunodeficiency virus infection. Endocr Pract. 1999;5:1–9. doi: 10.4158/EP.5.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Bhasin S, et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA. 2000;283:763–770. doi: 10.1001/jama.283.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhasin S, et al. Effects of testosterone replacement with a nongenital, transdermal system, Androderm, in human immunodeficiency virus-infected men with low testosterone levels. J Clin Endocrinol Metab. 1998;83:3155–3162. doi: 10.1210/jcem.83.9.5079. [DOI] [PubMed] [Google Scholar]
- 40.Dobs AS, et al. The use of a transscrotal testosterone delivery system in the treatment of patients with weight loss related to human immunodeficiency virus infection. Am J Med. 1999;107:126–132. doi: 10.1016/s0002-9343(99)00193-x. [DOI] [PubMed] [Google Scholar]
- 41.Grinspoon S, et al. Effects of androgen administration in men with the AIDS wasting syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129:18–26. doi: 10.7326/0003-4819-129-1-199807010-00005. [DOI] [PubMed] [Google Scholar]
- 42.Grinspoon S, et al. Effects of testosterone and progressive resistance training in eugonadal men with AIDS wasting. A randomized, controlled trial. Ann Intern Med. 2000;133:348–355. doi: 10.7326/0003-4819-133-5-200009050-00010. [DOI] [PubMed] [Google Scholar]
- 43.Storer TW, et al. A randomized, placebo-controlled trial of nandrolone decanoate in HIV-infected men with mild to moderate weight loss with recombinant human growth hormone as active reference treatment. J Clin Endocrinol Metab. 2005;90:4474–4482. doi: 10.1210/jc.2005-0275. [DOI] [PubMed] [Google Scholar]
- 44.Grunfeld C, et al. A 12-week randomized, placebo-controlled trial of oxandrolone in HIV-infected patients with weight loss. J Acquir Immune Defic Syndr. doi: 10.1097/01.qai.0000197546.56131.40. in press. [DOI] [PubMed] [Google Scholar]
- 45.Berger JR, et al. Oxandrolone in AIDS-wasting myopathy. AIDS. 1996;10:1657–1662. doi: 10.1097/00002030-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Kong A, Edmonds P. Testosterone therapy in HIV wasting syndrome: systematic review and meta-analysis. Lancet Infect Dis. 2002;2:692–699. doi: 10.1016/s1473-3099(02)00441-3. [DOI] [PubMed] [Google Scholar]
- 47.Rabkin JG, et al. A double-blind, placebo-controlled trial of testosterone therapy for HIV-positive men with hypogonadal symptoms. Arch Gen Psychiatry. 2000;57:141–147. doi: 10.1001/archpsyc.57.2.141. [DOI] [PubMed] [Google Scholar]
- 48.Grinspoon S, et al. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. J Clin Endocrinol Metab. 2000;85:60–65. doi: 10.1210/jcem.85.1.6224. [DOI] [PubMed] [Google Scholar]
- 49.Rabkin JG, et al. Testosterone versus fluoxetine for depression and fatigue in HIV/AIDS: a placebo-controlled trial. J Clin Psychopharmacol. 2004;24:379–385. doi: 10.1097/01.jcp.0000132442.35478.3c. [DOI] [PubMed] [Google Scholar]
- 50.Rabkin JG, et al. Testosterone therapy for human immunodeficiency virus-positive men with and without hypogonadism. J Clin Psychopharmacol. 1999;19:19–27. doi: 10.1097/00004714-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Miller K, et al. Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: a pilot study. J Clin Endocrinol Metab. 1998;83:2717–2725. doi: 10.1210/jcem.83.8.5051. [DOI] [PubMed] [Google Scholar]
- 52.Dolan S, et al. Effects of testosterone administration in human immunodeficiency virus-infected women with low weight: a randomized placebo-controlled study. Arch Intern Med. 2004;164:897–904. doi: 10.1001/archinte.164.8.897. [DOI] [PubMed] [Google Scholar]
- 53.Choi HH, et al. Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J Clin Endocrinol Metab. 2005;90:1531–1541. doi: 10.1210/jc.2004-1677. [DOI] [PubMed] [Google Scholar]
- 54.Reid IR, et al. Plasma testosterone concentrations in asthmatic men treated with glucocorticoids. Br Med J (Clin Res Ed) 1985;291:574. doi: 10.1136/bmj.291.6495.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reid IR, et al. Testosterone therapy in glucocorticoid-treated men. Arch Intern Med. 1996;156:1173–1177. [PubMed] [Google Scholar]
- 56.Crawford BA, et al. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab. 2003;88:3167–3176. doi: 10.1210/jc.2002-021827. [DOI] [PubMed] [Google Scholar]
- 57.Casaburi R, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 58.Schols AM, et al. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo controlled randomized trial. Am J Respir Crit Care Med. 1995;152:1268–1274. doi: 10.1164/ajrccm.152.4.7551381. [DOI] [PubMed] [Google Scholar]
- 59.Morley JE, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41:149–152. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 60.Snyder PJ, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 61.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–1098. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 62.Page ST, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 63.Kenny AM, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–M272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- 64.Sih R, et al. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 65.Ferrando AA, et al. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88:358–362. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- 66.Wittert GA, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58:618–625. doi: 10.1093/gerona/58.7.m618. [DOI] [PubMed] [Google Scholar]
- 67.Liverman CT, Blazer DG, editors. Testosterone and Aging: Clinical Research Directions. National Academies Press; Washington, DC: 2004. [PubMed] [Google Scholar]
- 68.Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl. 2001;22:718–731. [PubMed] [Google Scholar]
- 69.Calof O, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 70.Kearbey JD, et al. Pharmacokinetics of S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide in rats, a non-steroidal selective androgen receptor modulator. Xenobiotica. 2004;34:273–280. doi: 10.1080/0049825041008962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao W, et al. Comparison of the pharmacological effects of a novel selective androgen receptor modulator, the 5α-reductase inhibitor finasteride, and the antiandrogen hydroxyflutamide in intact rats: new approach for benign prostate hyperplasia. Endocrinology. 2004;145:5420–5428. doi: 10.1210/en.2004-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin D, et al. Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol Pharmacol. 2003;63:211–223. doi: 10.1124/mol.63.1.211. [DOI] [PubMed] [Google Scholar]
- 73.Gao W, et al. Selective androgen receptor modulator (SARM) treatment improves muscle strength and body composition, and prevents bone loss in orchidectomized rats. Endocrinology. 2005;146:4887–4897. doi: 10.1210/en.2005-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salvati ME, et al. American Association for Cancer Research Annual Meeting: 1996 March 27–31. Orlando, FL: 2004. Design, structural analysis, and biological profile of a novel series of AR antagonists. abstract 1948. [Google Scholar]
- 75.Bohl CE, et al. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci USA. 2005;102:6201–6206. doi: 10.1073/pnas.0500381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanada K, et al. Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis. Biol Pharm Bull. 2003;26 doi: 10.1248/bpb.26.1563. [DOI] [PubMed] [Google Scholar]
- 77.Gronemeyer H, et al. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 78.Katzenellenbogen BS, Katzenellenbogen JA. Biomedicine. Defining the “S” in SERMs. Science. 2002;295:2380–2381. doi: 10.1126/science.1070442. [DOI] [PubMed] [Google Scholar]
- 79.Kemppainen JA, et al. Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol. 1999;13:440–454. doi: 10.1210/mend.13.3.0255. [DOI] [PubMed] [Google Scholar]
- 80.He B, Wilson EM. Electrostatic modulation in steroid receptor recruitment of LXXLL and FXXLF motifs. Mol Cell Biol. 2003;23:2135–2150. doi: 10.1128/MCB.23.6.2135-2150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang CY, McDonnell DP. Evaluation of ligand-dependent changes in AR structure using peptide probes. Mol Endocrinol. 2002;16:647–660. doi: 10.1210/mend.16.4.0818. [DOI] [PubMed] [Google Scholar]
- 82.Sathya G, et al. Pharmacological uncoupling of androgen receptor-mediated prostate cancer cell proliferation and prostate-specific antigen secretion. Cancer Res. 2003;63:8029–8036. [PubMed] [Google Scholar]
- 83.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 84.Kelce WR, et al. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- 85.Waller CL, et al. Three-dimensional quantitative structure–activity relationships for androgen receptor ligands. Toxicol Appl Pharmacol. 1996;137:219–227. doi: 10.1006/taap.1996.0075. [DOI] [PubMed] [Google Scholar]
- 86.Christiansen RG, et al. Antiandrogenic steroidal sulfonylpyrazoles. J Med Chem. 1990;33:2094–2100. doi: 10.1021/jm00170a008. [DOI] [PubMed] [Google Scholar]
- 87.Hamann LG. Discovery and preclinical profile of a highly potent and muscle selective androgen receptor modulator (SARM); 227th National Meeting of the American Chemical Society Medicinal Chemistry Division: 2004 March 28–April 1; Anaheim, CA. 2004. MEDI-175. [Google Scholar]
- 88.Miyakawa M, et al. Patent WO 2004013104 (US patent 2005277660) Preparation of novel tetrahydroquinoline derivatives as androgen receptor agonists. 2004






