Introduction
With the rapid development of imaging techniques and better understanding of structural and functional pathology of the spine and spinal cord there has been a worldwide increase in the number of spine surgeries performed, particularly in specialized interdisciplinary spine centers. In addition to the congenital and acquired deformities of the spine and relatively rare spinal cord tumors, common degenerative spine disease within the aging general population contributes to a growing number of pathologies with myelopathies. This is important because antecedent myelopathy increases spinal cord risk during surgical treatment. The possibility of having functional neurophysiological assessment during spine and spinal cord surgery was introduced in 1970s by applying somatosensory evoked potentials (SEPs) as well as spinal evoked potentials [15, 21, 32, 33].
Meanwhile these modalities and continuous EMG recording have been enhanced by the addition of corticospinal motor pathway monitoring through the use of motor evoked potentials (MEPs) elicited by transcranial electrical stimulation [3]. The application of multimodal intraoperative monitoring (MIOM) became routine in several spine centers, being documented by publications about the specificity and sensitivity as well as clinical experience and outcome measurements during different spinal surgical procedures [6, 9, 13, 14, 17, 22, 24, 26, 27]. In this supplement on intraoperative monitoring the Spine Center of the Schulthess Clinic presents their experience with the application of MIOM during spine surgery analyzing 1,017 operations in the years 2000–2005. The patients for monitoring were selected out of 11,356 who received spine surgery at the institution during the study period. The indication for each monitoring procedure was discussed and established within the monitoring and surgical team. The detailed analysis of this large patient population resulted in a sensitivity of 89% and specificity of 99%. Sutter et al. [31] conclude that MIOM is an effective method of monitoring the spinal cord functional integrity during spine surgery and therefore can lead to reduction of neurological deficits and consequently improve postoperative results. An independent series of 206 thoracolumbar surgeries also presented in this supplement supports this conclusion [19].
Sala et al. [27] discussed in their recent publication of a historical control study the ethical limitation for performing prospective randomized studies to assess the efficacy of MIOM while clearly documenting that monitoring improves outcome after surgery for intramedullary spinal tumors.
The Spine Society of Europe (SSE) supported the development of MIOM by presentations of the results at its annual meetings and organized workshops to stimulate discussion and communication between the spine surgeons and clinical neuroscientists. The SSE has also introduced quality control management in spine surgeries by establishing Spine Tango, a web-based international registry that includes MIOM documentation (http://www.eurospine.org). Other international spine societies such as Scoliosis Research Society presented several original papers on MIOM at the 41st annual meeting in 2006 [2, 5, 23] illustrating its advantages for surgery of adolescent idiopathic and infantile scoliosis.
Recently, the International Society of Intraoperative Neurophysiology (ISIN) was founded to stimulate interdisciplinary communication and collaboration between surgeons, neurologists, neurophysiologists and anesthetists (http://www.ptsroma.it/isin).
The aim of a first consensus meeting on intraoperative monitoring during spine surgeries in Verona (28 September 2006) was to provide recommendations for the improvement and appropriate application of monitoring techniques during spine surgeries.
Experts in the field were invited for a meeting with the support of the European Spine Journal (Max Aebi, MD, Editor-in-chief, Marek Szpalski, MD, Supplement Editor) to summarize the current state-of-the-art and prepare current opinions and recommendations. This consensus statement represents a work in progress and as with all other recommendations or proposals, it must be updated as new information is gained.
MIOM rationale and nomenclature
Reviewing the literature on intraoperative monitoring makes it clear that application of a single method such as SEPs is not sufficient and that accounting for ascending and descending pathways of the spinal cord and nerve roots requires a multimodal approach. In order to facilitate communication among practitioners and surgeons as well as understanding of the underlying pathophysiology and to help surgeons to understand interpretations presented by the neurophysiologist during the operation, an eventual unification of nomenclature is desirable.
Evoked potential nomenclature has occasionally been misleading. For example, “neurogenic MEPs” peripheral nerve potentials evoked by spinal cord stimulation turned out not to be motor [8, 20, 35]. Nevertheless, terminology established by the International Federation of Clinical Neurophysiology (IFCN), American Clinical Neurophysiology Society (ACNS) and American Society of Neurophysiologic Monitoring (ASNM) is accurate, widely applied and acceptable [1, 4, 16, 34]. Surgeons and practitioners should be ideally familiar with established terms, including: D and I wave; muscle MEP; spinal, subcortical and cortical SEP; free-running and triggered EMG; compound muscle action potential (CMAP); compound nerve action potential (CNAP); F-response; H-reflex, etc. This may be particularly true if the monitoring is performed by technicians. However, the consensus group recommends that the monitoring be carried out by an appropriately trained neurologist or neurophysiologist to allow for open discussion.
Nomenclature should distinguish between the selective and the non-selective modalities. SEPs select the dorsal column somatosensory system. MEPs select the corticospinal motor system and only potentials evoked by brain stimulation qualify. Descending corticospinal volleys are properly referred to as D and I waves as originally named and muscle responses as muscle MEPs [7].
Spinal cord stimulation non-selectively activates all ascending and descending systems and the evoked spinal cord, nerve or muscle potentials are neither MEPs nor SEPs [7, 18]. Although lower motor neurons ultimately mediate muscle responses, their possible excitation from extrapyramidal pathways including branches of antidromically activated dorsal column axons must be recognized. The terms spino-spinal EP and spino-muscular EP are suggested to set these potentials apart as being non-selective.
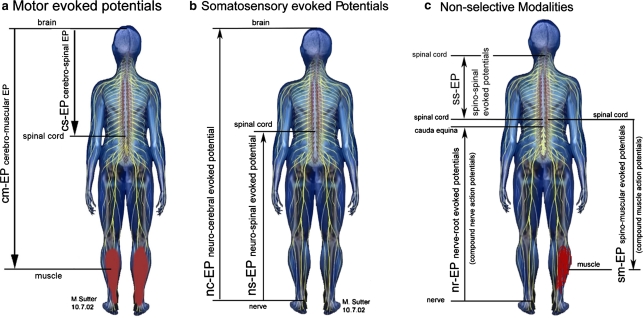
The consensus group recognized that alternative terminology based on specifying stimulation and recording sites as originally proposed by Tamaki [32, 33] and modified by Sutter et al. [31] can also be acceptable (Fig. 1; Table 1). Practitioners and surgeons preferring this approach should be thoroughly familiar with the associated terms while realizing that they are not widely used. Whether or not this alternative will eventually supplant more established nomenclature remains to be seen.
Fig. 1.
a Principles of stimulation and recording sites of MEP. b Principles of stimulation and recording sites of SEP. c Principles of non-selective modalities
Table 1.
Alternative abbreviations and descriptions of the different modalities according to Fig. 1
| Abbreviation | Description |
|---|---|
| cm-EP | Cerebro-muscular evoked potential |
| cs-EP | Cerebro-spinal evoked potential |
| ns-EP | Neuro-spinal evoked potential |
| nc-EP | Neuro-cerebral evoked potential |
| sm-EP | Spino-muscular evoked potential |
| ss-EP | Spino-spinal evoked potential |
| nr-EP | Nerve-root evoked potential |
MIOM principles and methodology
Figure 1 presents the principles of motor, somatosensory and non-selective evoked potentials indicating the stimulating and recording sites. The neural structures to be stimulated and the recording sites (brain, spinal cord, nerves and muscles) are to be chosen in accordance with any anticipated injury. Monitoring with evoked potentials is to be done on both sides (of the body: MEPs and SEPs) separately as well as proximal and distal to the site of risk to distinguish systemic changes due to anesthesia, temperature and other pathologically related changes due to ischemia or surgery. Monitoring upper and lower limb evoked potentials can further improve this distinction while also providing brachial plexus and arm peripheral nerve protection [19].
MIOM technology is not yet formally standardized and a variety of approaches exist. Deletis [7] and Macdonald [18] recently provided extensive reviews of contemporary MEP methodologies. In this supplement, Sutter et al. [31] and Macdonald and Al Zayed [19] present their current respective spine MIOM protocols and Sala et al. [25] present their current approach to monitoring intramedullary spinal cord tumor surgery. It was agreed that intravenous anesthesia and omission of neuromuscular blockade after intubation is important to successful MIOM (Table 2).
Table 2.
Proposed anesthesia protocol for intraoperative monitoring during spine surgery
| Proposed anesthesia protocol in MIOM | |
|---|---|
| Remifentanyl | 0.2–0.9 μg/kg/min |
| Propofol | 3–10 mg/kg/h |
| Ketamin | 2–6 mg/kg/h |
| Recuronium | Only for intubation |
| Sevoflurane, N2O, etc. | Should not be used |
The consensus group discussed the efficacy and safety of invasive spinal monitoring techniques and agreed that they are valuable in selected cases. However, it was recognized that epidural D wave alterations have been observed as an artifact of curve correction [36]. Thus, motor predictions should probably not rely solely on epidural D waves during scoliosis surgery; subarachnoid recordings might not be subject to this problem. In addition, D waves and spino-spinal EP’s can miss or delay the detection of spinal cord ischemia that is more rapidly and reliably detected by muscle MEPs [18]. Hence, muscle MEP monitoring should always be done.
Invasive D wave monitoring was unanimously considered an essential component of intramedullary spinal cord tumor surgery monitoring, for which muscle MEPs alone are insufficient [7, 18]. This is because intramedullary dissection can selectively disrupt the spinal cord’s intrinsic supportive motor systems, leading to muscle MEP loss without corticospinal tract or alpha motor neuron injury. In these special circumstances, D wave preservation indicating corticospinal tract integrity usually predicts good long-term motor outcome. This can allow completing the tumor resection despite muscle MEP loss.
No consensus was reached on the importance of invasive techniques during other spinal surgeries in which this special mechanism of muscle MEP deterioration is not known to occur. Some members found the addition of invasive recordings to be important because of their rapid acquisition and stability [10, 29, 32]. Some other members found it difficult to justify their added complexity and possible risks because modern non-invasive techniques appear sufficient when including muscle MEPs that are also rapidly acquired although less stable [18, 19, 24, 26, 27, 36]. Consensus on this point will need further study.
The group’s combined experience of a few thousand invasive recordings revealed only two complications (meningitis and broken electrode tip). Other possible hemorrhagic, traumatic or infectious adverse effects have not yet been reported [32]. Thus, these techniques appear sufficiently safe but require due care and expertise.
Intraoperative monitoring team, education
The consensus group agreed that close collaboration between spine surgeon, the responsible neurologist/neurophysiologist, the anesthetist and possibly involved technician is important to interpret the intraoperative, electrophysiological results in order to reach a shared decision on a possible alteration of the surgical procedure. The understanding of the basic principles of the monitoring techniques by the spine surgeon on one hand and the understanding of the spine pathology and the surgical approaches by the neurologist on the other hand are prerequisites to facilitate the shared decision in case of altered potentials indicating possible risk or danger for damage of spinal cord and/or nerve roots.
It is recommended that the responsible person on the monitoring side should be an medical doctor (MD) with subspecialty education in clinical neurophysiology and additional education on intraoperative monitoring. As in some countries the profession of clinical neurophysiologist has been established, it therefore could be considered as an equivalent to a neurologist. Although technicians can be helpful during the procedure, their education does not supply the background to bear the responsibility of interpreting the results.
In addition, the consensus group recommends that spine units/centers who decide to introduce and implement MIOM in their institution allow the dedicated neurologist/neurophysiologist to obtain the necessary additional education and experience through practical collaboration with already well-established monitoring teams for an appropriate period of time. Three to 6 months (according to educational background) is considered as a reasonable period. The consensus group also recommends the ISIN to develop an accreditation system for spine centers integrating intraoperative monitoring into their routine procedures.
Indications for multimodal intraoperative monitoring
The consensus group is of the opinion that MIOM is indicated or recommended in all spinal surgical procedures bearing a potential risk of damaging neural structures. The high sensitivity and specificity of MIOM during the different surgical procedures as presented in this supplement [10–12, 28–31] document its advantages. It has been clearly shown that MEP monitoring improves outcome after surgery for intramedullary spinal tumors [27].
The consensus group agreed that MIOM can be recommended for the following spinal pathologies:
corrections of spinal deformities with scoliosis greater than 45°,
corrections of congenital spine anomalies,
resections of intramedullary and extramedullary tumors, and
extensive anterior and/or posterior decompressions in spinal stenosis in cervical, thoracic and lumbar spine causing myelopathies and functional disturbance of cauda equina and/or individual nerve roots.
Also the consensus group was of the opinion that there is sufficient scientific evidence to propose the above recommendations, however, not enough to establish legally binding guidelines. Currently it is up to the responsibility of the spinal surgeon and the neurologist to establish the indication for intraoperative monitoring according to the individual cases receiving treatment. However, the current body of knowledge justifies the development and establishment of MIOM as routine procedure in spine centers to improve the surgical results and reduce the risk of potential damage to the neural structure.
Future perspectives
The consensus group is of the opinion and recommends the establishment of MIOM as a routine procedure in spine centers dealing with severe spinal disorders in which surgical procedures could lead to damage of neural structures. The current body of knowledge makes the wake-up test to monitor the correction of spinal deformities obsolete.
Close collaboration of the different international scientific societies such as the Spine Society of Europe, the ISIN, the Scoliosis Research Society, the International Society for Study of the Lumbar Spine and the Cervical Spine Research Society could facilitate interdisciplinary communication as well as the establishment of the monitoring units in spine centers. It is also recommended that the ISIN takes this paper as a “study in progress” and elaborates on the development of quality management and standards for intraoperative monitoring as well as educational standards and accreditation.
As the specificity and sensitivity of MIOM is well-established, the design of historical studies as presented by Sala et al. [27] should be initiated to gain more sound information on the efficacy of monitoring procedures to reduce neurological complications (as obviously performance of prospective randomized trials is limited by ethical considerations).
Acknowledgments
The authors would like to acknowledge the support of Charles McCammon from the research department of Schulthess Clinic for organizing the consensus meeting and preparing the manuscript.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.(1994) Guideline eleven: guidelines for intraoperative monitoring of sensory evoked potentials. American Electroencephalographic Society. J Clin Neurophysiol 11(1):77–87 [PubMed]
- 2.Auerbach JD, Schwartz DM, Drummond DS, Jones KJ, Flynn JM, El-Gazzar Y, McPartland T, Bowe A, Laufer S, Pizzutillo P, Bowen R, Dormans JP (2006) Detection of impending neurologic injury during surgery for adolescent idiopathic scoliosis: a comparison of transcranial motor and somatosensory evoked potential monitoring in 1121 consecutive cases, in scoliosis research society 41st annual meeting and pre-meeting course SRS: Monterey California. Sept 13–16
- 3.Boyd SG, Rothwell JC, Cowan JM, Webb PJ, Morley T, Asselman P, Marsden CD. A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. J Neurol Neurosurg Psychiatr. 1986;49(3):251–257. doi: 10.1136/jnnp.49.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke D, Nuwer MR, Daube J, Fischer C, Schramm J, Yingling CD, Jones SJ. Intraoperative monitoring. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:133–148. [PubMed] [Google Scholar]
- 5.Cheh G, Lenke LG, Kim YJ, Daubs MD, Padberg A, Kuhns CA, Stobbs G, Hensley M (2006) Loss of spinal cord monitoring signals in children during thoracic kyphosis correction with spinal osteotomy: why does it occur and what should you do? In Scoliosis Research Society 41st annual meeting and pre-meeting course, Monterey [DOI] [PubMed]
- 6.Cronin AJ. Spinal cord monitoring. Curr Opin Orthop. 2002;13:188–192. doi: 10.1097/00001433-200206000-00006. [DOI] [Google Scholar]
- 7.Deletis V. Intraoperative neurophysiology and methodologies used to monitor the functional integrity of the motor system. In: Deletis, editor. Neurophysiology in neurosurgery. California: Academic; 2002. pp. 25–51. [Google Scholar]
- 8.Deletis V, Sala F. The role of intraoperative neurophysiology in the protection or documentation of surgically induced injury to the spinal cord. Ann NY Acad Sci. 2001;939:137–144. doi: 10.1111/j.1749-6632.2001.tb03620.x. [DOI] [PubMed] [Google Scholar]
- 9.Deletis V, Sala F. Intraoperative neurophysiological monitoring during spine surgery: an update. Curr Opin Orthop. 2004;15:154–158. doi: 10.1097/01.bco.0000127314.99341.ad. [DOI] [Google Scholar]
- 10.Eggspühler A, Sutter M, Grob D, Jeszenszky D, Dvorak J (2007) Multimodal intraoperative monitoring during surgery of spinal deformities in 217 patients. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 11.Eggspühler A, Sutter M, Grob D, Jeszenszky D, Porchet F, Dvorak J (2007) Multimodal intraoperative monitoring (MIOM) during cervical spine surgical procedures in 246 patients. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 12.Eggspühler A, Sutter M, Grob D, Porchet F, Jeszenszky D, Dvorak J (2007) Multimodal intraoperative monitoring (MIOM) during surgical decompression of thoracic spinal stenosis in 36 patients. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 13.Iwasaki H, Tamaki T, Yoshida M, Ando M, Yamada H, Tsutsui S, Takami M. Efficacy and limitations of current methods of intraoperative spinal cord monitoring. J Orthop Sci. 2003;8(5):635–642. doi: 10.1007/s00776-003-0693-z. [DOI] [PubMed] [Google Scholar]
- 14.Kothbauer K, Deletis V, Epstein F (1998)Motor evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures, http://www.aans.org/journals/online_j/may98/4-5-1 [DOI] [PubMed]
- 15.Kurokawa T. Spinal cord action potentials evoked by epidural stimulation of the spinal cord––a report of human and animal record. Jpn J Electroenceph Electromyogr. 1972;1:64–66. [Google Scholar]
- 16.Leppanen RE, Abnm D, American Society of Neurophysiological M (2005) Intraoperative monitoring of segmental spinal nerve root function with free-run and electrically-triggered electromyography and spinal cord function with reflexes and F-responses. A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput 19(6):437–461 [DOI] [PubMed]
- 17.Luk KD, Hu Y, Wong YW, Cheung KM. Evaluation of various evoked potential techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26(16):1772–1777. doi: 10.1097/00007632-200108150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald DB. Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput. 2006;20(5):347–377. doi: 10.1007/s10877-006-9033-0. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald DB, Al Zayed Z (2007) Four-limb muscle motor evoked potential and optimized somatosensory evoked potential monitoring with decussation assessment: results in 206 thoraculumbar spine surgeries. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 20.Minahan RE, Sepkuty JP, Lesser RP, Sponseller PD, Kostuik JP. Anterior spinal cord injury with preserved neurogenic ‘motor’ evoked potentials. Clin Neurophysiol. 2001;112(8):1442–1450. doi: 10.1016/S1388-2457(01)00567-3. [DOI] [PubMed] [Google Scholar]
- 21.Nash CL, Brodkey JS, Croft TJ. A model for electrical monitoring of spinal cord function in scoliosis patients undergoing correction. J Bone Joint Surg Am. 1972;54A:197–198. [Google Scholar]
- 22.Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96(1):6–11. doi: 10.1016/0013-4694(94)00235-D. [DOI] [PubMed] [Google Scholar]
- 23.Olaverri JCR, De Blas G, Vallespir GP, Burgos J, Hevia E, Vicente J, Sanpera I, Domenech P, Maruenda J, Ignacio R (2006.) Triggered electromyographic threshold in the intercostals muscle to evaluate the accuracy of high thoracic pedicle screw placement. In Scoliosis Research Society 41st annual meeting and pre-meeting course, Monterey
- 24.Owen JH. The application of intraoperative monitoring during surgery for spinal deformity. Spine. 1999;24(24):2649–2662. doi: 10.1097/00007632-199912150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Sala F, Faccioli F, Lanteri P, Gerosa M (2007) Surgery for intramedullary spinal cord tumors: the role of intraoperative neurophysiological monitoring. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 26.Sala F, Krzan MJ, Deletis V. Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv Syst. 2002;18(6–7):264–287. doi: 10.1007/s00381-002-0582-3. [DOI] [PubMed] [Google Scholar]
- 27.Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, Bricolo A. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58(6):1129–1143. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]
- 28.Sutter M, Eggspühler A, Grob D, Jeszenszky D, Benini A, Porchet F, Muller A, Dvorak J (2007) The validity of multimodal intraoperative monitoring (MIOM) in surgery of 109 spine and spinal cord tumors. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 29.Sutter M, Eggspühler A, Grob D, Jeszenszky D, Porchet F, Muller A, Dvorak J (2007) The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: a prospective study of 1017 cases. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 30.Sutter M, Eggspühler A, Grob D, Porchet F, Jeszenszky D, Dvorak J (2007) Mutlimodal intraoperative monitoring (MIOM) during lumbosacral surgical procedures in 409 patients. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 31.Sutter M, Eggspühler A, Muller A, Dvorak J (2007) Multimodal intraoperative monitoring: an overview of proposed methodology based on 1017 cases. Eur Spine J (suppl.) [DOI] [PMC free article] [PubMed]
- 32.Tamaki T (2007) History and development of intraoperative monitoring during spine surgery. Eur Spine J
- 33.Tamaki T, Yamashita T, Kobayashi H (1972) Spinal cord monitoring. Jpn J Electroenceph Electromyogr 1:196
- 34.Toleikis JR. Intraoperative monitoring using somatosensory evoked potentials. A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2005;19(3):241–258. doi: 10.1007/s10877-005-4397-0. [DOI] [PubMed] [Google Scholar]
- 35.Toleikis JR, Carlvin AO, Shapiro DE, Schafer MF. The use of dermatomal evoked responses during surgical procedures that use intrapedicular fixation of the lumbosacral spine. Spine. 1993;18(16):2401–2407. doi: 10.1097/00007632-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Ulkatan S, Neuwirth M, Bitan F, Minardi C, Kokoszka A, Deletis V. Monitoring of scoliosis surgery with epidurally recorded motor evoked potentials (D wave) revealed false results. Clin Neurophysiol. 2006;117(9):2093–2101. doi: 10.1016/j.clinph.2006.05.021. [DOI] [PubMed] [Google Scholar]