Abstract
To describe different currently available tests of multimodal intraoperative monitoring (MIOM) used in spine and spinal cord surgery indicating the technical parameters, application and interpretation as an easy understanding systematic overview to help implementation of MIOM and improve communication between neurophysiologists and spine surgeons. This article aims to give an overview and proposal of the different MIOM-techniques as used daily in spine and spinal cord surgery at our institution. Intensive research in neurophysiology over the past decades has lead to a profound understanding of the spinal cord, nerve functions and their intraoperative functional evaluation in anaesthetised patients. At present, spine surgeons and neurophysiologist are faced with 1,883 publications in PubMed on spinal cord monitoring. The value and the limitations of single monitoring methods are well documented. The diagnostic power of the multimodal approach in a larger study population in spine surgery, as measured with sensitivity and specificity, is dealt with elsewhere in this supplement (Sutter et al. in Eur Spine J Suppl, 2007). This paper aims to give a detailed description of the different modalities used in this study. Description of monitoring techniques of the descending and ascending spinal cord and nerve root pathways by motor evoked potentials of the spinal cord and muscles elicited after transcranial electrical motor cortex, spinal cord, cauda equina and nerve root stimulation, continuous EMG, sensory cortical and spinal evoked potentials, as well as direct spinal cord evoked potentials applied on 1,017 patients. The method of MIOM, continuously adapted according to the site, stage of surgery and potential danger to nerve tissues, proved to be applicable with online results, reliable and furthermore teachable.
Keywords: Spine surgery, Intraoperative monitoring, Method
Introduction
In major spine centers around the world the multimodal intraoperative monitoring procedures performed by experienced neurophysiologist became an integrated part of the spine team on one side to reduce neurological complications occurring during the spine surgery and improving the surgical results on the other side. Reviewing the published scientific work from the different centres it is clear that one method such as SEP to monitor the function of spinal cord and nerve roots is not sufficient and the multimodal approach account for the monitoring of the ascending and descending pathways of the spinal cord. In order to facilitate the scientific communication and understanding of the underlying physiology and to help the surgeons to interpret the presented results by the neurophysiologist during the operation a unification of the methods description and the nomenclature among the experts and different group is advisable. The foundation of the International Society for Intraoperative Monitoring might play a catalyzing role in this important process.
Somatosensory evoked potentials (SEP) were introduced for IOM in the 1970s [12]. It is a well-established fact [5, 13, 15, 16] that SEP alone is not a reliable tool for assessment of the descending motor pathways: damage will therefore not be primarily recognised by SEP. After basic physiological research in the 1950s [14] motor evoked potentials (MEP) with spinal cord recording were introduced for clinical diagnostic examinations [11] and for monitoring scoliosis surgery [3] in the 1980s. Tamaki et al. [18] and Kurokawa [10] introduced direct spinal cord stimulation and recording techniques (spinal cord evoked potentials, SCEP) in the early 1970s. Spinal electrodes allow the simultaneous recording of spinal sensory evoked potentials (SSEP) and cerebral sensory evoked potentials and yield various sensory level diagnoses. Cerebro-muscular and spino-muscular evoked potentials reveal information about the functioning of the upper and lower motoneuron as well as neuromuscular transmission. They are recorded by compound muscle action potentials (CMAP). This technique has become possible using modern electrical stimulator techniques, which can perform short repetitive trains of single stimuli and with the introduction of intravenous anaesthesia such as propofol in combination with short acting opioids or ketamine [22].
Study aims
Systematic description of the currently available tests to be applied during the multimodal intraoperative monitoring:
Presentation of parameters and settings for the different tests to reach the optimal recording.
Patient population, materials and methods
As in our institution annually approximately 2,000 patients are operated on the spine the selection criteria to apply intraoperative monitoring are decided and prepared by the spine team, i.e., the spine surgeon, the neurologist, and the anaesthetist. Influenced by the expertise from V. Deletis, T. Tamaki and H. Nishiura as well as at the early stage J. Herdmann, in March 2000 the first author consequently introduced and since performed the MIOM and following the experience enhanced by the second author and the remaining spine team on 1,017 consecutive patients who underwent a total of 4,731 h of MIOM to evaluate any neural deficit that may have occurred during spine surgery until December 2005.
Materials
The advancement of the hardware allowed the neurophysiologist to adapt the stimulating and recording parameters and settings to analyse the different potentials online. In order to obtain reproducible and reliable potentials and or information about pathological changes, the settings and their understanding are most decisive.
For the study two Keypoint® 8-channel workstations and two Keypoint® 4m/4c devices from Medtronic-Dantec™ (Denmark) were used with integrated electrical stimulators and custom software. Stimulation of peripheral nerves in SEP and recording of compound muscle action potentials after cerebral or spinal cord stimulation was carried out using surface electrodes (Medtronic-Dantec 901L0202; Ambu™ 720 01-SC, Denmark), except in the case of EMG from the anal sphincter and bulbocaverenosus muscles, for which monopolar or bipolar needle electrodes were used (Medtronic-Dantec, 9013L0252 resp., 9013S0021). For cerebral motor stimulation as well as for the recording of cerebral sensory evoked potential monopolar needle electrodes (Medtronic-Dantec, 9013L0252) were placed at C3′ and C4′ according to the international EEG 10-20 System. Bipolar spinal electrodes were placed subarachnoidal (Inter Medical™, IMC-KG-102, Japan) or epidural (Inter Medical™, IMC-KG-102, Japan; Inomed™ GmbH, FSR03/100, Germany) for electrical spinal stimulation or recording. They were introduced either preoperatively by lumbar puncture with a Tuohy needle 18G (Portex™ Ltd. UK) or intraoperatively by the surgeon. Monopolar needle (Medtronic-Dantec, 9013L0252) and mono- or bibolar nerve stimulator devices (Inomed™ GmbH, 79331, Germany) were used for direct nerve, root, tumour or pedicle screw stimulations.
Mounting of electrodes and cables was carried out at the same time as induction of anaesthesia. On average, it prolonged the presurgical procedure by 5–15 min, depending on whether a spinal electrode had to be inserted by lumbar puncture for monitoring the spinal cord at the onset of surgery. The anaesthesia protocol is outlined in Table 1.
Table 1.
Anaesthesia protocol for intraoperative monitoring during spine surgery used in this study
| Protocol of anaesthesia | ||
|---|---|---|
| Remifentanyl (Ultiva) | Induction 0.1–0.2 μg/kg/min for 3 min | Maintain dose 0.4 (0.2–0.8) μg/kg/min, mainly dependent on heart frequency and blood pressure |
| Propofol (Disoprivan) | Bolus 0.2–0.25 mg/kg 10 mg/kg/h for 10 min, then 5 mg/kg/h until start of the operation | 4 (3–12) mg/kg/h during operation |
| Ketamin (Ketalar) | Not used for induction | 2–6 mg/kg/h, instead of, or in combination with propofol |
| Recuronium (Esmerol) | 0.6–0.9 mg/kg | Only for intubation |
| Sevofluran, N2O,... | Seldom used | Not used |
Method of multimodal intraoperative monitoring
Main principles of evoked potentials
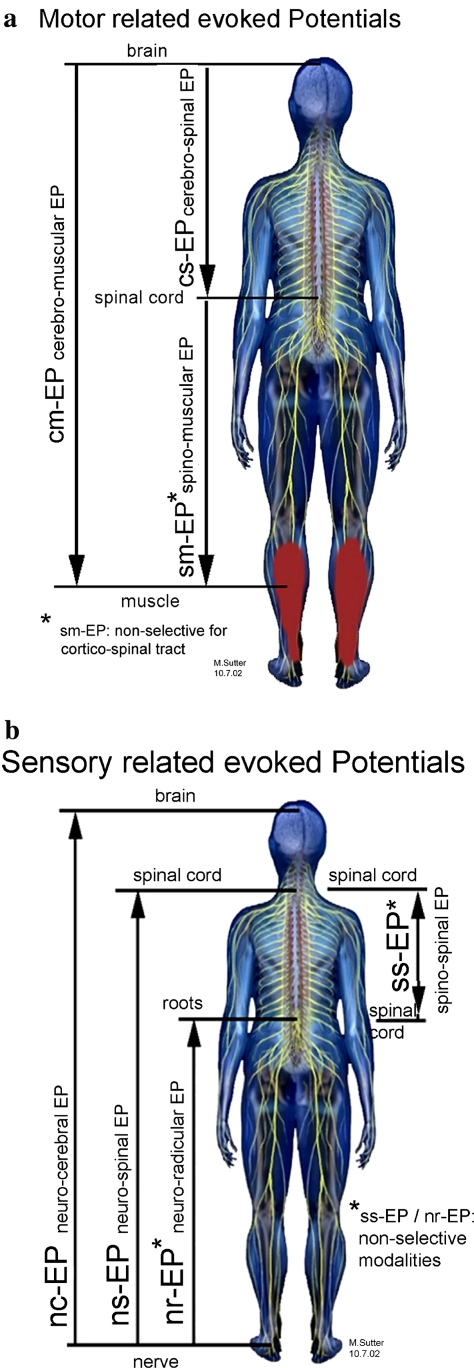
As specifically for multimodal use, conventional nomenclature of evoked potentials has misleading terms (e.g., “cortical” MEP’s are actually elicitated subcortically) [14] and misleading abbreviations [e.g., “SSEP” may be used in somato-sensory (cortical)] evoked potentials as well as for spinal sensory evoked potentials, a new nomenclature with clear labelling of stimulation and recording sites is suggested here and was strictly used in the present study (Fig. 1).
Fig. 1.
a Principles of stimulation and recording sites of decending pathways. b Principles of stimulation and recording sites of ascending pathways
The neural structures to be stimulated and the recording sites (skull/brain, spinal cord, nerves, muscles) were chosen in accordance with any anticipated injury caused by surgical procedure. Monitoring was always done on both the right and left sides and proximal as well as distal to the site of risk, to distinguish systemic changes (anaesthetic, perfusion, temperature, etc.) from direct surgery-related changes. The same scalp needle electrodes used for transcranial electrical motor stimulation in cm-EP (cerebro-muscular evoked potentials; previous nomenclature: MEP, Br-CMAP, etc.) and cs-EP (cerebro-spinal evoked potentials; previous nomenclature: cortico-spinal MEP, D-wave Monitoring) were also used for recording nc-EP (neuro-cerebral evoked potentials; previous nomenclature: SEP, SSEP, CSEP). The same epidural or subarachnoidal spinal electrode can be used rostrally for recording ns-EP (neuro-spinal evoked potentials; previous nomenclature: SSEP), ss-EP (spino-spinal evoked potentials; previous nomenclature: SCEP spinal cord evoked potentials) and also for stimulating sm-EP (spino-muscular evoked potentials; previous nomenclature: spinal-CMAP’s, sc-MEP’s) as well as for control recordings for cs-EP (cerebro-spinal evoked potentials). The same caudal spinal electrode, primarily used for monitoring the corticospinal tract in cs-EP, was also used as a control for recording the ns-EP and as the stimulation site in ss-EP. In cervical spine surgery, the recording of compound muscle action potentials (CMAP) from peripheral muscles in cm-EP and sm-EP yields information in the upper extremities about anterior root innervation and at the legs about the corticospinal tract. The peripheral nerves and dermatomes were selected to provide information about the dorsal sensory roots and the dorsal columns at the site of risk.
The anaesthesia protocol is outline in Table 1. In our institution over the past 5 years we made the experience that the induction and maintain of anaesthesia are sufficient with intravenously administered Propofol or Ketamin and the control of pain perception by the group of opioids for example remifentanyl; muscle relaxant was only given for intubation.
Motor evoked potentials
Electrical scalp stimulation can activate the corticospinal tract and evoke a synchronised “D”—(direct) wave followed by various “I”—(indirect, transsynaptically activated) waves which are recorded along the spinal cord [14]. As a result of general anaesthesia, recording of peripheral muscle activation is harder, but has become possible after introduction of a short train of transcranial electrical stimulation [20]. Intraoperative motor evoked potentials can also be elicited by trains of transcranial magnetic stimulation [21], but electrical stimulation with scalp needle electrodes leads to more reliable and reproducible potentials and is easier to perform. Transcranial magnetic stimulation was therefore not used intraoperatively in the present study.
Transcranial anodal motor cortex stimulation of C3′–C4′ and C4′–C3′ with simultaneous recording of all contralateral muscles was usually used in the present study. The stimulation and recording parameters are shown in Table 2. Monitoring with cm-EP was done without application of muscle relaxants. As cm-EP may lead to significant muscle twitches, the surgeon was informed before performing this test. For allowing quantitative analysis of the cm-EP, supramaximal motor stimulation was used if available and repeated train stimulation with averaging the CMAP’s was performed. Cm-EP was never performed in patients with a history of epilepsy or acute or subacute strokes as it may provoke seizures. The latency, amplitude and shape of averaged cm-EP were analysed for monitoring. For possible trend analysis, recording filters were not changed during the whole surgical procedure. The simultaneous recording of two to four muscle pairs was always performed to identify surgery-related changes. Depending on the spine surgical procedure, one proximal muscle pair was examined as a control and one to three muscle pairs for analysing root functions, of which at least one pair on the legs was used for monitoring corticospinal tract. The tests were usually repeated at 5-min intervals for possible trend analysis and at any further time upon surgical demand. Changes in cm-EP were considered to be pathological if there was an increase in latency of more than 10% or a decrease in amplitude of more than 50%. If cs-EP and ss-EP remained unchanged, local decreased cm-EP were considered to indicate lower motorneuron dysfunction. In the present study, cm-EP recording of different muscle groups was used together with cs-EP and sm-EP for level diagnosis of lesions of the corticospinal tract, considering that anterior horn cells are located 0.5 to 1.5 level above the spine segment. In root or plexus monitoring, the combined use of ns-EP, nc-EP and rm-EP monitoring was used to identify and localise nerve and/or root lesions.
Table 2.
Cerebro-muscular evoked potentials elicited by transcranial electrical stimulation (cmEP)
| Stimulation | Monopolar needles | Stimulus (anode) | ISI | Intensity | Duration | Rate of repetition |
|---|---|---|---|---|---|---|
| Skalp | C3′–C4′ (left) C4′–C3′ (right) | Repeated train of 5 (2–6) stimuli | 2.5 ms (2–3) | 100 mA (50–200) | 0.5 ms (0.2–0.8) | 1/s |
| Recording | Electrodes | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Muscles of interest, synchronous | 2 surface electrodes/muscle | CMAP | 0.05–3 kHz | 0.05 mV/D | 10 ms/D | 4–10 |
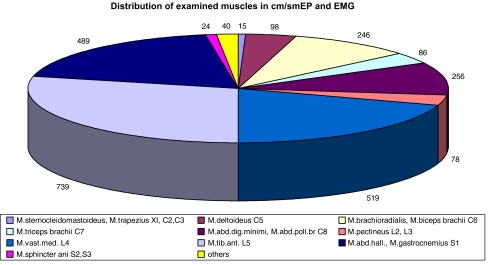
Spino-muscular and root-muscular or nerve-muscularEP (s/r/nm-EP) (Tables 3, 4), were elicited at the diagnostic level of the spinal cord using an epidural or subarachnoidal bipolar spinal electrode. The potentials were recorded from the muscles using surface electrodes. The same recording pattern as that described for cm-EP was used. Direct stimulation of the spinal cord was done with care; i.e., the current was increased slowly and gradually and was limited to submaximal stimulation because of progressively severe muscle twitches. Sm-EP was never done on the cranio-cervical junction because of the considered risk of accidental stimulation of vagus nuclei. Depending on the lateral positioning of the spinal electrode and non-selective activation of corticospinal tract, spinal stimulation was seen to elicit muscular potentials asymmetric to the limbs. Only changes from the initial baseline were used for monitoring. Evidence of preserved sm-EP to the legs was considered to be helpful (but not determining) in the differentiation between rostral corticospinal tract lesions and systemic or metabolic disorders and in combination with cm/cs-EP, ss-EP and ns/nc-EP was considered helpful in true level diagnosis. The sm-EP test setting described in Table 3 was also used for selective root, tissue or pedicle screw stimulation tests (Table 4) done by the surgeons with monopolar needle or bipolar hand-held stimulators. As there are no normative conduction values for these tests, only an increase of the sm-EP latencies exceeding 10% and/or a decrease of sm-EP amplitudes of more than 50%, compared to baseline, were considered pathological. In fusion surgery, stimulation of pedicle screws was considered to provide useful information regarding the distance of the screw to the neural tissue in an unaffected root. A threshold stimulation level of more than 8 mA was considered normal, 5–8 mA critical and less than 5 mA pathological, indicating that there was not enough distance between the screws and the neural tissue. Care was taken to ensure that the most appropriate muscle was measured as shown in Fig. 3. In tumour surgery, s/r/nm-EP exited by monopolar hand-held stimulators was mainly used to identify (threshold value usually 0.2–3 mA) or exclude (threshold value >12 mA) functional neural tissue.
Table 3.
Spino-muscular evoked potentials (sm-EP)
| Stimulation | Bipolar spinal electrode | Stimulus (cathode) | ISI | Intensity | Duration | Rate of repetition |
|---|---|---|---|---|---|---|
| Spinal cord | Epidural or subarachnoidal | Repeated single (or train) stimuli | −(2.5 ms) | (0.2–15) mA | 0.2 ms | 1/s |
| Recording | Electrodes | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Muscles of interest, synchronous | 2 surface electrodes/muscle | CMAP | 0.05–3 kHz | 0.05 mV/D | 10 ms/D | 2–10 |
Table 4.
Root- and nerve-muscular evoked potentials (r/nm-EP)
| Stimulation | Bi- or monopolar stimulator | Stimulus (cathode) | ISI | Intensity | Duration | Rate of repetition |
|---|---|---|---|---|---|---|
| Root, nerve | Screw, tumour, ... | Repeated single stimuli | – | 0.5–30 mA | 0.2 ms | 5.9/s |
| Recording | Electrodes | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Muscles of interest, synchronous | 2 surface electrodes/muscle | CMAP | 0.05–3 kHz | 0.05 mV/D | 10 ms/D | 2–10 |
Fig. 3.
Distribution of recorded muscles in cm/smEP and EMG
In cs-EP, as in cm-EP, transcranial electrical anodal stimulation was carried out using the same monopolar needle electrodes C3′–C4′ for primarily monitoring the right corticospinal tract and C4′–C3′ for primarily monitoring the left corticospinal tract. Stimulation and recording parameters are shown in Table 5. “D-waves” (indicating direct activation of the corticospinal tract) were used for monitoring. Caudal to the T12 level, cs-EP became progressively polyphasic with decreasing amplitudes indicating compound nerve action potentials (CNAP) of the roots at the level of the conus medullaris and cauda equina and were not considered to represent information on the corticospinal tract. The latency (L) and baseline to negative-peak-amplitude (A) of the averaged D-waves were analysed for quantitative trend analysis. Tests of each corticospinal tract were usually repeated at 5 min intervals for quantitative trend analysis, or at anytime upon surgical demand. D-waves were usually highly stable potentials during the whole surgical procedure and allowed monitoring of (the most important) corticospinal tract, even in patients with severe neurological deficits and in those for whom intraoperative transient muscle relaxation was necessary (precluding cm-EP monitoring). Gradual reductions of the amplitude to 50% during long lasting operations were not considered to be associated with new neurological deficits, but sudden changes of as little as 5–10% of the amplitude in combination with deformity of the potential were discussed with the surgeon as a possible indicator of postoperative central paresis; persistent loss of D-waves at the end of the operation was considered to lead to limb para- or tetraplegia, depending on the level and laterality of the alteration. Usually epidural spine electrodes were inserted intraoperatively by the surgeon. In ventral surgical procedures, or in need of monitoring the dorsal surgical approach, a subarachnoidal spinal electrode was introduced preoperatively by lumbar puncture. Use of rostral and caudal spine electrodes were used to distinguish cortical or systemic affection from pure spinal cord damage and was considered most important in spinal vascular disorders or spinalis anterior syndrome. Monitoring with cs-EP was done without relevant muscle twitches and without disruption to the surgeon.
Table 5.
Cerebro-spinal evoked potentials (cs-EP)
| Stimulation | Monopolar needles | Stimulus (anode) | ISI | Intensity | Duration | Rate of repetition |
|---|---|---|---|---|---|---|
| Skalp | C3′–C4′ (left) C4′–C3′ (right) | Repeated single stimuli | – | 100 mA (50–200) | 0.5 ms | 3/s |
| Recording | Electrode | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Spinal cord epidural or subarachnoidal | Bipolar spinal electrode | D-wave | 0.5–3 kHz | 5 uV/D | 2 ms/D | 20–200 |
Monitoring using ss-EP (Table 6), was recorded with the rostral spinal electrode after caudal spinal cord stimulation with the same electrodes used for cs-EP. Stimulation at the cranio-cervical site was not done due to considered vagus nuclei stimulation. Similar to D-wave monitoring, spinal cord evoked potentials were usually stable, although they were considered non-selective but mainly reflect activity of sensory related tracts as submaximal stimulation was done [6, 19]. A reduction of amplitude of more than 50% or increase of latencies of more than 10% was considered to indicate postoperative spinal cord disorder. Slow and careful increase of initial stimulation from 0.5 mA to maximum 15 mA, usually with a repetition rate of 10/s, was used for ss-EP affecting tonic muscle contractions.
Table 6.
Spino-spinal evoked potentials (ssEP)
| Stimulation | Bipolar spinal electrode | Stimulus (cathode) | ISI | Intensity | Duration | Rate of repetition |
|---|---|---|---|---|---|---|
| Spinal cord | Epidural or subarachnoidal | Repeated single stimuli | – | 0.5–15 mA | 0.2 ms | 10/s |
| Recording | Bipolar spinal electrode | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Spinal cord | Epidural or subarachnoidal | SCEP | 2–3 kHz | 0.2 uV/D | 1 ms/D | 50–200 |
EMG recordings (Table 7) were done with the same surface muscle electrodes as those used for cm/sm-EP. During intraoperative irritation or compression of nerve roots, so-called myotonic discharge (repetitive pseudorhythmic firing of motor units) was analysed as an indicator of possible nerve root dysfunction [2, 8].
Table 7.
EMG settings
| Recording | Electrode | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Muscles of interest, synchronous | Bipolar surface | MUAP | 0.01–5 kHz | 0.2 mV/D | 100 ms/D | – |
Sensory evoked potentials
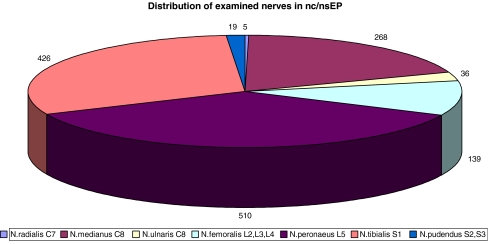
Sensory evoked potentials were elicited mainly by stimulation of the median, ulnar, radial, tibial, peroneal and femoral nerve trunks or with dermatomal stimulation to monitor root and dorsal tract function. Tibial or peroneal nc-EP were additionally used to monitor dorsal column function. Recording was usually done transcranially using the same scalp needle electrodes C3′–C4′ (and C4′–C3′) as those used to stimulate cm/cs-EP. Spinal subarachnoidal or epidural electrodes were used for the diagnosis of affected level and to distinguish systemic changes.
nc-EP (Table 8) were recorded transcranially with monopolar needles (C3′/C4′ and C4′/C3′) after stimulating peripheral nerves (right and left). Latencies and amplitudes were measured for trend analysis. Increase of latencies of more than 10% and/or decrease in amplitudes of more than 50% were considered to be pathological. Somatosensory evoked potentials were still widely used and helpful for monitoring root and dorsal tract function as well as maintain the level of anaesthesia and hemodynamics. The combination of several nc-EP allowed affected levels to be diagnosed.
Table 8.
Neuro-cerebral evoked potentials (nc-EP)
| Stimulation | Electrode | Stimulus (cathode) | ISI | Intensity | Duration | Rate of repetition |
|---|---|---|---|---|---|---|
| Nerve | Bipolar surface | Repeated single stimuli | – | 10–50 mA | 0.2 ms | 5.9/s |
| Recording | Monopolar needle | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Skalp | C3′–C4′; C4′–C3′; Cz-Fz | Cortical and subcortical SEP | 0.05–0.3 kHz | 1 µV/D | 10 ms/D | 200 (100–1,000) |
ns-EP and nr-EP (Table 9) were observed after the stimulation of the peripheral nerves and recorded with the spinal electrodes primarily used for cs-EP. They were performed simultaneously with nc-EP. ns-EP recordings mainly helped with sensory level diagnostics, distinguishing alterations from roots to the dorsal tract and also helped to differentiate systemic from surgery-related changes. There are no normative values for ns-EP. The same scalp electrodes which were used for the electrical stimulation of motor cortex and simultaneously for the recording of the sensory-evoked potentials were also used to monitor the level of anaesthesia by recording EEG curve (electroencephalography) (Table 10). Monitoring burst-suppression EEG activity, respectively, the BIS-analysis is helpful for fine-tuning of the level of the anaesthesia as related to the monitored blood pressure to allow for the optimal recording of evoked potentials.
Table 9.
Neuro-spinal and nerve-root evoked potentials (ns/nrEP)
| Stimulation | Electrode | Stimulus | ISI | Intensity | Duration | Rate of repetition |
|---|---|---|---|---|---|---|
| Nerve | Bipolar surface or needle | Repeated single stimuli | – | 10–50 mA | 0.2 ms | 5.9/s |
| Recording | Bipolar spinal electrode | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| Spinal cord, cauda equina | Epidural or subarachnoidal | SSEP (rostral of epiconus) CNAP (caudal of conus medullaris) | 0.05–3 kHz | 5 µV | 10 ms/D | 100–1,000 |
Table 10.
EEG settings for analysis burst-suppression pattern and bispectral index
| Recording | Electrode | Potential | Filters | Sensitivity | Sweep | Averaging |
|---|---|---|---|---|---|---|
| C3′–C4′ | Monopolar needle | Cont. recording | 0.5–100 Hz | 0.1 mV/D | 200 ms/D | – |
Discussion
The first attempt to monitor the spinal cord function has been introduced by Patton and Amassian [14] for recording cortiocospinal tract in animal models. This study presented for the first time the so-called D-wave (D-wave/Direct activation of pyramidal cells). Tamaki et al. [18] introduced the spinal cord-evoked potentials by direct stimulation and recording by applying spinal electrodes. His pioneering work was introduced in the operating room and became a routine procedure during complex spine surgeries.
Merton and Morton [11] introduced electrical stimulation of motor cortex in humans, which was later replaced by the painless method of magnetic stimulation [1].
Deletis [4] consequently used the multimodal monitoring procedure during surgical resection of the spine tumors and documented the importance of the close collaboration between spine surgeon [7] and the neurophysiologist. The systematic analysis has been presented by Kothbauer [9] as a source of information and practical applicability of the multimodal intraoperative monitoring.
The current body of literature clearly indicates the advantages of multimodal approach to monitor the function of ascending and descending pathway during spine surgery. This multimodal approach clearly replaced application of single methods such as SEP or EMG.
In our institution the intraoperative monitoring was introduced in the early 1990s but only in the late 1990s by introduction and development of the hardware and the improved tests the intensive collaboration between the neurophysiologist and surgeons became useful in the daily routine while the information about function of the spinal cord and or nerve roots were presented to the surgeon instantly or as an online assessment. With the practical experience on 1,017 complex monitoring we are presenting the systematic overview most of the currently available methods which we have learned by personal communication from Tamakai and Deletis and further developed according to the needs of our spine surgeons by adapting the parameters and settings for the different procedures. Originating the standard nomenclature of the neurophysiology literature we present here a proposal or discussion of the different modalities primarily referred to the stimulation and the recording sites. Such a standardization could serve as a baseline for comparison of the results of the different studies joint effort for improvement of the multimodal intraoperative monitoring. Also this systematic overview and presentation of tests setting and parameters should facilitate the introduction of multimodal intraoperative monitoring in the spine centres as well as improve the learning curve of the neurophysiologist to offer the surgeons not only reliable and reproducible potentials but also the appropriate interpretation of the results.
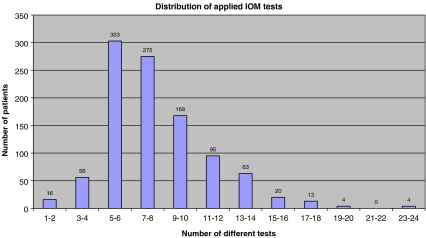
Figures 2, 3 and 4 document the distribution of the number of different tests being applied particular monitoring. The number of muscles and nerves being examined is a clear indicator of the multimodality. We have learned that the interpretation of the results should only be related to the particular recordings of a respective motor or sensory function of particular nerve root and/or part of the spinal cord (we can only interpret what we really truly measure).
Fig. 2.
Distribution of applied IOM tests
Fig. 4.
Distribution of stimulated nerves in nc/nsEP
Acknowledgments
Dr. Lote Medicus for her financial support of the development of MIOM at the Schulthess Clinic. DG, AB, FP, DJ, FL, FK, VB spine surgeons in our Spine unit for their support and collaboration. Dave O’Riordan and Charles McCammon for help with the manuscript. Anne Mannion Ph.D. for the critical review of the manuscript.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 2.Beatty RM, McGuire P, Moroney JM, Holladay FP. Continuous intraoperative electromyographic recording during spinal surgery. J Neurosurg. 1995;82(3):401–405. doi: 10.3171/jns.1995.82.3.0401. [DOI] [PubMed] [Google Scholar]
- 3.Boyd SG, Rothwell JC, Cowan JM, Webb PJ, Morley T, Asselman P, Marsden CD. A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. J Neurol Neurosurg Psychiatry. 1986;49(3):251–257. doi: 10.1136/jnnp.49.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deletis V. Intraoperative monitoring of the functional integrity of the motor pathways. In: Devinsky O, Beric A, Dogali M, editors. Advances in neurology: electrical and magnetic stimulation of the brain. New York: Raven; 1993. pp. 201–214. [PubMed] [Google Scholar]
- 5.Deletis V, Sala F. Intraoperative neuropphysiological monitoring during spine surgery: an update. Curr Opin Orthop. 2004;15:154–158. doi: 10.1097/01.bco.0000127314.99341.ad. [DOI] [Google Scholar]
- 6.Deutsch H, Arginteanu M, Manhart K, Perin N, Camins M, Moore F, Steinberger AA, Weisz DJ. Somatosensory evoked potential monitoring in anterior thoracic vertebrectomy. J Neurosurg. 2000;92(2 Suppl):155–161. doi: 10.3171/spi.2000.92.2.0155. [DOI] [PubMed] [Google Scholar]
- 7.Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993;79(2):204–209. doi: 10.3171/jns.1993.79.2.0204. [DOI] [PubMed] [Google Scholar]
- 8.Gunnarsson T, Krassioukov AV, Sarjeant R, Fehlings MG. Real-time continuous intraoperative electromyographic and somatosensory evoked potential recordings in spinal surgery: correlation of clinical and electrophysiologic findings in a prospective, consecutive series of 213 cases. Spine. 2004;29(6):677–684. doi: 10.1097/01.BRS.0000115144.30607.E9. [DOI] [PubMed] [Google Scholar]
- 9.Kothbauer KF. Motor evoked potential monitoring for intramedullary spinal cord tumor surgery. In: Deletis V, Shils JL, editors. Neurophysiology in neurosurgery. London: Academic; 2002. pp. 74–89. [Google Scholar]
- 10.Kurokawa T. Spinal cord action potentials evoked by epidural stimulation of the spinal cord—a report of human and animal record. Jpn J Electroenceph Electromyogr. 1972;1:64–66. [Google Scholar]
- 11.Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285(5762):227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- 12.Nash CL, Brodkey JS, Croft TJ (1972) A model for electrical monitoring of spinal cord function in scoliosis patients undergoing correction. J Bone Joint Surg Am (54A):197–198
- 13.Owen JH. The application of intraoperative monitoring during surgery for spinal deformity. Spine. 1999;24(24):2649–2662. doi: 10.1097/00007632-199912150-00012. [DOI] [PubMed] [Google Scholar]
- 14.Patton HD, Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17(4):345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- 15.Sala F, Lanteri P, Bricolo A. Motor evoked potential monitoring for spinal cord and brain stem surgery. Adv Tech Stand Neurosurg. 2004;29:133–169. doi: 10.1007/978-3-7091-0558-0_4. [DOI] [PubMed] [Google Scholar]
- 16.Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, Bricolo A. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58(6):1129–1143. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]
- 17.Sutter M, Eggspühler A, Muller A, Dvorak J (2007) Multimodal intraoperative monitoring: an overview of proposed methodology based on 1017 cases. Eur Spine J (Suppl) [DOI] [PMC free article] [PubMed]
- 18.Tamaki T, Yamashita T, Kobayashi H. Spinal cord monitoring. Jpn J Elektraenceph Elektromyogra. 1972;1:196. [Google Scholar]
- 19.Tamaki T, Noguchi T, Takano H, Tsuji H, Nakagawa T, Imai K, Inoue S. Spinal cord monitoring as a clinical utilization of the spinal evoked potential. Clin Orthop Relat Res. 1984;184:58–64. [PubMed] [Google Scholar]
- 20.Taylor BA, Fennelly ME, Taylor A, Farrell J. Temporal summation—the key to motor evoked potential spinal cord monitoring in humans. J Neurol Neurosurg Psychiatry. 1993;56(1):104–106. doi: 10.1136/jnnp.56.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ubags LH, Kalkman CJ, Been HD, Koelman JH, Ongerboer Visser BW. A comparison of myogenic motor evoked responses to electrical and magnetic transcranial stimulation during nitrous oxide/opioid anesthesia. Anesth Analg. 1999;88(3):568–572. doi: 10.1097/00000539-199903000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Zentner J. Motor evoked potential monitoring during neurosurgical operations on the spinal cord. Neurosurg Rev. 1991;14(1):29–36. doi: 10.1007/BF00338189. [DOI] [PubMed] [Google Scholar]