Abstract
In a prospective study of 109 patients with tumor of the spine MIOM was performed during the surgical procedure between March 2000 and December 2005. To determine the sensitivity and specificity of MIOM techniques used to monitor spinal cord and nerve root function during surgical procedure of spinal tumors. MIOM become an integrated procedure during surgical approach to intramedullar and extramedullar spine tumors. The combination of monitoring ascending and descending pathways may provide more sensitive and specific results than SEP alone giving immediate feedback information regarding any neurological deficit during the operation. Intraoperative sensory spinal and cerebral evoked potential combined with EMG recordings and motor evoked potential of the spinal cord and muscles were evaluated and compared with postoperative clinical neurological changes. One hundred and nine consecutive patients with spinal tumors of different aetiologies were monitored by the means of MIOM during the entire surgical procedure. Eighty-two patients presented true negative findings while two patients monitored false negative, one false positive and 24 patients true positive findings where neurological deficits after the operation were present. All patients with neurological deficit recovered completely or to pre-existing neurological situation. The sensitivity of MIOM applied during surgery of spinal tumors has been calculated of 92% and specificity 99%. Based upon the results of the study MIOM is an effective method of monitoring the spinal cord and nerve root function during surgical approach of spinal tumors and consequently can reduce or prevent the occurrence of postoperative neurological deficit.
Keywords: Spine surgery, Tumors, Intraoperative monitoring, Sensitivity, Specificity
Introduction
Reference spine centres are commonly confronted with patients with spinal tumors. According to the origin three major groups can be distinguished:
Intramedullar tumors (such as ependymoma and astrocytoma)
Extramedullar, intradural tumors (such as neurinoma, meningioma)
Epidural tumors (chordomas, teratomas, hemangiomas and carcinomas metastases).
The management of spinal tumors requires a close collaboration of spine surgeons and neurologists specialised in the intraoperative neurophysiological monitoring.
Reports about application of intraoperative SEP monitoring are of limited value [17] while SEPs are monitoring only the ascending sensory pathways and give little or no information about the descending motor pathways. The relatively high proportion of false negative cases [17] clearly documents the limitation of intraoperative SEP monitoring alone.
The inadequacy of SEPs for monitoring of the functional integrity of motor pathways in the spinal cord has been documented in several reports [6, 8, 9]. Sala [11] explains the misleading use of the term “false negative” to describe the onset of postoperative paraplegia in spite of preserved SEPs. Such an event should not be described as a “false negative” result since SEPs are not aimed to test the corticospinal pathways. Most likely using MEPs instead of, or in combination with SEPs, would have transformed those so-called “false negative” into “true positive” results [11].
The introduction of spinal electrodes for direct stimulation and recording of spinal cord evoked potentials but also for monitoring the corticospinal tract after transcranial motor cortex stimulation [1, 16] was a major advancement for evaluating functional integrity of motor deficits. The transcranial stimulation technique allows for the recording of the D-wave by a spinal electrode placed epi- or subdurally. This potential is a highly reliable parameter for monitoring the functional integrity of the motor pathways intraoperatively while representing a population of fast conducting fibres of the corticospinal tract.
The assessment protocol to monitor the functional integrity of spinal cord during surgery of intramedullary spinal cord tumors has been advanced by Epstein in the 1980s. He introduced aggressive guided excision by intraoperative neurophysiological monitoring with attempted gross total resection of the tumor [2–5]. The advent of modern microsurgical techniques has yielded a significant change in the approach to intramedullary spinal cord tumors, following the pioneering work of Epstein which has dramatically improved the outcome for those patients harbouring these tumors, particularly those in the paediatric population [13]. The same group of authors recommend in cases of benign intramedullary tumor of spinal cord aggressive MEP monitoring guided resection as soon as possible before the onset of neurological deterioration.
Kothbauer [7] reported the advantage of application of motor evoked potential monitoring for intramedullary spinal cord tumor surgery in a series of 100 consecutive procedures.
The correlation of changes in transcranial motor evoked potentials during intramedullar spinal cord tumor resection with postoperative motor function has been documented and such quantitative intraoperative monitoring data may help to minimize postoperative motor deficit by avoiding or correcting excessive spinal cord tumor manipulation and modifying surgical technique during tumor resection [10].
The presented studies are mainly observational prospective clinical series as prospective randomized controlled trials are unlikely to be performed. Sala [11] clearly expressed the reasons, while neurosurgeons or spine surgeons who systematically rely on neurophysiological techniques during surgery would be reluctant from both an ethical and medicolegal perspective to withhold intraoperative neurophysiological assistance from a designated control group. Showing the benefit of MIOM is limited to historical control studies as performed by Sala [12] in 2006. He compared the outcome of a group of 50 patients with and without MIOM after surgery for intramedullar spinal cord tumors and could demonstrate that the patients operated with the assistance of MIOM had outcomes which were statistically significantly better. Consequently the system of labelling specific techniques as standard guideline or option can be less effectively applied to the intraoperative monitoring [11].
The aim of the study
To analyze the specificity and sensitivity for intraoperative multimodal monitoring in surgery of spine tumors.
Correlate clinical outcome with the intraoperative monitoring findings.
Patient population and method
Out of 1,017 patients who underwent spinal surgery between March 2000 and December 2005, 109 patients have been diagnosed with spinal tumor. The patients were 60 females and 49 males with a mean age of 51 years (range 11–86 years).
According to the type and location of the tumors 23 patients belonged to the group of intramedullary tumors, 41 cases were intradural-extramedullary and in 45 patients the tumor was located epidural (Table 1).
Table 1.
The distribution of tumors in the examined population of 109 patients
| Distribution of tumors | |||
|---|---|---|---|
| Location | Total | Aetiology | Frequency |
| Epidural | 45 | Aneurymatic cyst | 3 |
| Carcinom metastases | 10 | ||
| Cavernous angioma | 1 | ||
| Chondroma | 2 | ||
| Chordoma | 8 | ||
| Epidermoid cyst | 1 | ||
| Fibrous dysplasia | 1 | ||
| Glomus tumor | 2 | ||
| Hemangiopericytoma | 3 | ||
| Lipoma | 2 | ||
| Liposarcoma | 1 | ||
| Neurofibroma | 1 | ||
| Osteoblastoma | 4 | ||
| Plasmocytoma | 2 | ||
| Teratoma | 4 | ||
| Intradural extramedullary | 41 | Enchondroma | 1 |
| Epidermoid cyst | 1 | ||
| Ganglioneuroma | 1 | ||
| Gangliopericytoma | 1 | ||
| Chronic leptomeningitis | 5 | ||
| Lipoma | 1 | ||
| Meningeoma | 13 | ||
| Neurinoma | 14 | ||
| Neurofibroma | 4 | ||
| Intramedullar | 23 | Ependymoma | 10 |
| Lipoma | 1 | ||
| Neurinoma | 1 | ||
| Syringomyelia | 9 | ||
| Teratoma | 2 | ||
All monitorings were performed by two experienced neurologists with 10 years of clinical experience in neurophysiology (first two authors). The monitoring modalities and selection of muscles and nerves to be recorded respectively stimulated were applied to clinical situation and any needs that occurred during surgical procedure.
The details of the monitoring method as applied at the spine centre of Schulthess Clinic are described elsewhere by Sutter et al. [14, 15].
Results
The surgical procedures of the 109 patients with spine and spinal cord tumors were planned and performed according the pre-existing pathology and expected difficulties after clinical examination of the neurologist and spine surgeon and MRI-findings to plan intraoperative monitoring accordingly. The monitoring modalities and tests applied during the surgery are summarized in Table 2. The average time dedicated to monitoring was 5.3 h (ranging from 1.6 to 18.3 h) the longest monitoring time was required for a total resection of Chordoma growing between C2 and C4 with anterior and posterior fusion between C1 and C5.
Table 2.
Tests applied in the patient population (n = 109) with spinal tumors
| Monitoring modality | Monitorings applied | Baseline recordings | |||
|---|---|---|---|---|---|
| Out of 109 cases | Mean tests* per patient | normal potential | abnormal potential | no potentials | |
| cm-EP | 108 (99%) | 2.7 | 40 | 68 | 0 |
| sm-EP | 22 (20%) | 2.9 | 22 (NVM*) | 0 | |
| cs-EP | 67 (61%) | 1.2 | 24 | 37 | 6 |
| ss-EP | 15 (14%) | 1 | 15 (NVM) | 0 | |
| nc-EP | 99 (91%) | 1.5 | 36 | 62 | 1 |
| ns-EP | 55 (50%) | 1.5 | 55 (NVM) | 0 | |
| BCR, BAR | 15 (14%) | 1 | 15 (NVM) | 0 | |
| AEP | 1 (1%) | 2 | 1 | 0 | 0 |
| EMG | 91 (83%) | 2.7 | No spontaneous activity | ||
aTests: recorded muscle pairs or stimulated nerve pairs in a given modality
cm-EP cerebro-muscular evoked potentials, cs-EP cerebro-spinal evoked potentials, ns-EP neuro-spinal evoked potentials, nc-EP neuro-cerebral evoked potentials, sm-EP spino-muscular evoked potentials, ss-EP spino-spinal evoked potentials, BCR bulbo-cavernous reflex, BAR bulbo-anal reflex, NVMnormative value missing
In the examined group there was one false positive and 24 true positive findings which means that in 24 surgical procedures based on the monitoring technique, a postoperative neurological deterioration was predicted and also the surgical technique and procedure adapted accordingly. Eighty-two monitorings were true negative while in two patients false negative results have been obtained (Table 3).
Table 3.
Detailed description of two false negative findings in patients operated on spine tumors
| False negative cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Region | Pathology | Surgery | Duration | IOM modalities | IOM-baseline | IOM-changes | Neurological deterioration | Duration | Recovery |
| Z.M.,f, 62 years | L1 | Mama-CA-metastasis, fracture | Vertebrectomy fusion vertebroplasty | 5 h | cmPect.,VM,TA-EP EMGPect., VM,TA nPNc-EP |
All available | None | Sensory deficit L2 | 7 days | Completely |
| C.B., f, 70 years | C2-C4 | Chordoma | Decompression, and fusion, dorso-ventro-dorsal | 17 h | csT8EP cmTA,DM,ADMEP smTA,DM,ADMEP nMN,TNcEP nMN,TNsT8EP cont EMGTA,DM,ADM |
All available, but csEP and ncEP pathologic | Slowly reduction of all amplitudes | Sensory radiculopathy C5 left | 5 days | Completely |
Using the standard formula, the sensitivity of intraoperative monitoring in the group of spinal tumor has been calculated by 92%, the specificity 99%. By applying the 95% confidence interval (CI) the sensitivity ranged between 73.4 and 98.6% while CI of specificity ranged from 92.5 and 99.9%. (Table 4)
Table 4.
Detailed description of a false positive finding in a patient operated on for spinal tumor
| False positive case | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Region | Pathology | Surgery | Duration | IOM modalities | IOM-baseline | IOM-changes | Expected neurological deficit |
| P.M., f, 53 years | C0-T1 | Developmental tumor with Arnold-Chiari Malformation | Dekompression C1–C2 from dorsal | 4.5 h | cmBR,ADM-EP nMN,TNc-EP EMG BR,ADM |
Normal potentials | Alteration of nMNcEP both sides left nMN,TNcEP | Ataxia left side |
In the examined group the two false negative cases have to be described in detail. One case was a 62 year-old female with L1 metastases and pathological fracture due to breast cancer. The surgeon performed vertebrectomy with vertebroplasty and fusion, an operation lasting 5 h. The IOM modalities have been performed accordingly with cerebro-muscular evoked potentials from pectineus, vastus medialis, tibialis anterior muscles as well as continuous EMG of pectineus, vastus medialis and tibialis anterior muscles including the neuro-cerebral evoked potentials elicited by stimulation of the peroneal nerve. At the baseline as well as during the entire procedure the potentials were available and unchanged, however postoperatively the patient presented a sensory deficit in the distribution of L2 dermatoma which recovered completely within 7 days. This case of false negative finding documents the difficulty of monitoring the L2 nerve root and even the electromyography of the pectineus muscle showed no changes. However all but one sensory nerve root of the cauda were intact.
The other false negative case was a 70 year-old female with a progressive chordoma from C2 to C4 having already undergone two previous operations in another institution. The surgeon started decompression and tumor resection from the dorsal approach and continued in supine position to complete macroscopic total tumor vertebrectomy. Finally ventral and additional extended dorsal stabilisation was done with the appropriate technique (Fig. 1).
Fig. 1.
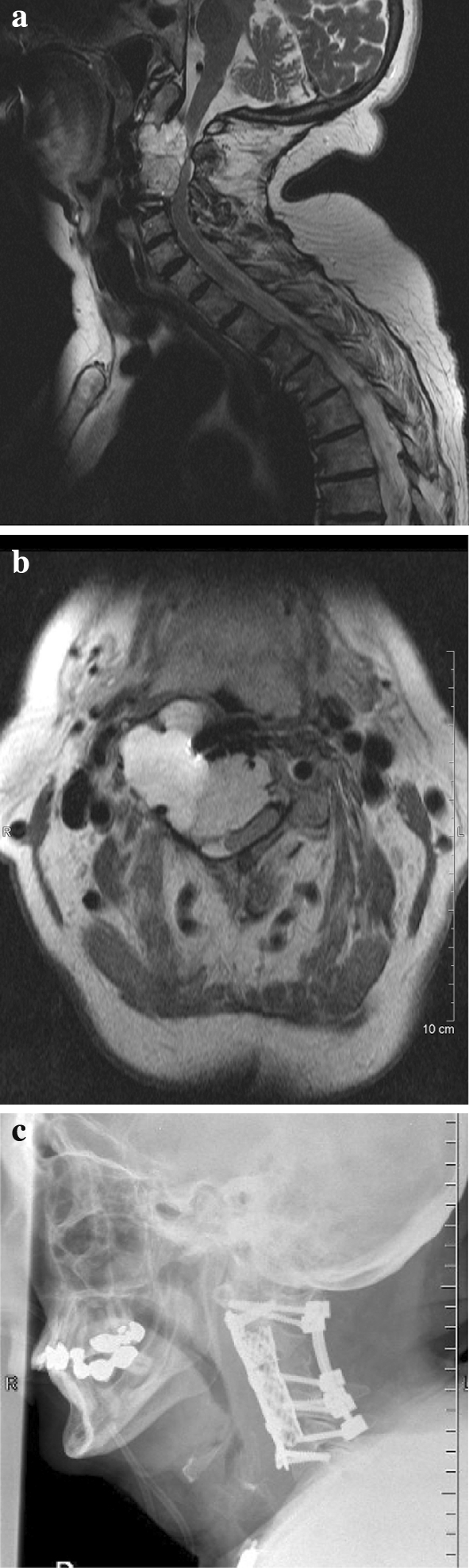
a Sagital MRI documentation of case C.B., f, 70 years with progressive growing chordoma C2–C4 and paraspasticity after two previous operations on the same level. b Axial MRI documenting the huge extension of the tumor with compression and dislocation of the spinal cord and right vertebral artery. c Postoperative X-ray showing dorsal and ventral stabilisation C1–C5
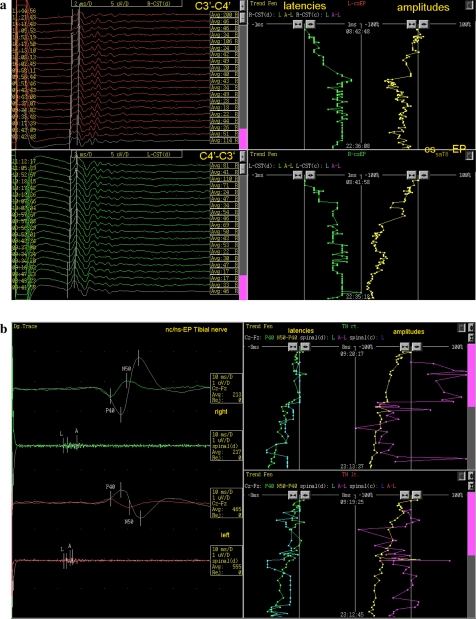
The entire surgical time was 17 h with high degree of surgical difficulties. At the baseline all potentials have been available but cerebro-spinal-evoked potentials and the neuro-cerebral potentials showed already pathological findings at the baseline. During the extremely demanding procedure for the surgeon and the neurophysiologist, the cerebro-spinal, cerebro-muscular, spino-muscular, neuro-cerebral as well as EMG recordings were performed on both the upper and lower extremities including monitoring from the epidural electrodes placed caudally to the region being operated. During the entire procedure the neurophysiologist/neurologist was present and communicated the monitoring results to the surgeon to adapt the surgical procedure accordingly and allow for the gross tumor resection following the principle of monitoring guided surgery as proposed by Epstein, Kothbauer and Sala [5, 7, 11]. During the procedure there was a gradual reduction of all amplitudes (Fig. 2). However the multimodal approach and power of interpretation did not reach pathological cut-off limits which urge to stop the resection of the tumor which tends to regrow if not resected completely.
Fig. 2.
a Trend analysis of cerebro-spinal evoked potentials. Left of the picture shows the pathological polyphasic D-waves from the beginning, indicating chronic demyelinisation of the corticospinal tract on both sides. During the operation, as shown on the right, significant alterations of the latencies and amplitudes of the D-waves were observed leading to adaption of the surgical approach. b Trend analysis of neuro-spinal and neuro-cerebral evoked potentials of right and left tibial nerves showing continuous reduction of amplitudes on both sides due to systemic drug, vascular and temperature effects
Postoperatively a deficit of the left sensory upper brachial plexus was documented however recovered completely within 5 days. This case documents the multimodal monitoring allowed the surgeon to remove the tumor completely without damaging the spinal cord, the nerve root or the vertebral arteries. However it documents that the results obtained can only be attributed to the recording sites. The sensory portion of the C5 nerve root can not be technically satisfactorily monitored. In the presented case the major concerns were focused on the spinal cord and the motor nerve roots at the operated region.
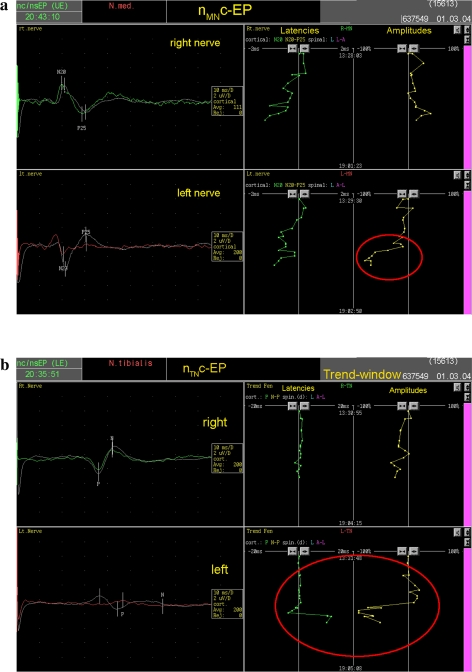
Alternatively, the false positive case occurred during a 4.5-h operation of developmental tumor of the craniocervical junctions combined with Arnold-Chiari malformation. The surgeon performed a posterior decompression followed by fusion. The IOM modalities have been chosen accordingly eliciting the cerebro-muscular evoked potentials to brachioradialis and abductor digiti minimi and neuro-cerebral evoked potentials from median nerve combined with continuous EMG of brachioradialis and abductor digiti minimi. At the baseline all potentials were normal however during the course of operation there was a continuous alteration in the neuro-cerebral evoked potentials of the left median and left tibial nerves (Fig. 3). According to his finding the neurologist was expecting a sensory deficit in the upper extremities possibly an ataxia which was fortunately not found by the postoperative clinical examination as the patient’s neurological exam was completely normal.
Fig. 3.
a, b Dorsal tract monitoring with neuro-spinal and neuro-cerebral evoked potential of median and tibial nerves with trend analysis of latencies and amplitudes. During the operation left median and left tibial nerve showed a significant alteration (red circles) indicating postoperative ataxia on the left arm and leg
The true positive cases based upon the continuous multimodal monitoring are described in detail in the Table 5. The clinical and histological diagnosis as well as the duration of the surgical procedures document the severity of the cases and the potential risks to the neurostructures which could occur in the tumor resection. The close collaboration between the neurologist and the spine surgeon following the pioneering work of Epstein by the means of monitoring guided surgery (Gross tumor resection) was applied. In 13 cases an onset or deterioration of pre-existing radiculopathy have been foreseen and confirmed by the clinical examination, however, all the operated patients recovered within hours till several months to the pre-existing neurological status. Two patients in this group died 2 and 8 months after the operation as a result of dissemination of carcinoma with multiple metastases.
Table 5.
Detailed description of 24 true positive findings in patients operated on for spine tumors
| True positive cases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Region | Pathology | Surgery | Duration (h) | IOM modalities | IOM-baseline | IOM-changes | Neurological deterioration | Duration | Recovery | |
| T.J., m, 55 years | e | L4-S2 | Carcinoma metastasis | Decompression, complete tumorexcision | 4.8 | cmEP, ncEP, in situ stimulation, BCR | Pathological cmEP and ncEP | Progressive alteration of all cmEP leg right | Deterioration of pre-existing radiculopathy L4&5 right | Exitus letalis after 2 months | No |
| E.D., f, 50 years | e | L3&4 | Carcinoma metastasis | Complete tumorcorpectomy ventral, fusion dorsal | 6.5 | csEP, cmEP, smEP, ncEP, nsEP, cont EMG | All available | Progressive deterioration of cmEP and smEP left leg | Radiculopathy L4&5 left | 5 months | Exitus letalis after 8 months |
| S.F., m, 59 years | e | L3 | Carcinoma metastasis | Complete tumorexcision and fusion dorsal | 4.2 | csEP, cmEP, nsEP | All available | deterioration of cmEP | Proximal weakness of legs | 2 days | complete |
| T.W., m, 56 years | e | C0-C3 | Hemangiopericytoma, recurrence | Decompression of myelon, partial tumorresection | 9.3 | csEP, cmEP, ncEP, in situ stimulation | All available | Alteration of cmEP trapecius muscle left | Mild accessory nerve paresis | 4 days | complete |
| Z.N., m, 67 years | e | L1-S1 | lipomatosis | Decompression and complete excision | 4 | csEP, cmEP, smEP, nsEP | All available | Loss of ncEP peroneal nerve left | Sensible radiculopathy S1 left | 2 weeks | complete |
| B.M., m, 44 years | e | L4-lumbosacral plexus | liposarcoma | Complete excision from dorsal | 4.3 | cmEP, ncEP, in situ stimulation, BCR | All available | Loss of cmEP TA&AH left, loss of ncEP PN&TN left | Ischiadic palsy left | 12 h | complete |
| S.S., f, 33 years | id-em | L2-S3 | ependymoma | Complete resection from dorsal | 7 | cmEP, in situ stimulation, ncEP, | All available | Deterioration of cmEP VM left | Quadriceps-paresis left | 1 day | complete |
| K.S., f, 26 years | id-em | S2-S5 | gangliopericytoma | Complete resection from dorsal | 7 | cmEP, smEP, ncEP, in situ stimulation, BCR | All available | Deterioration of cmEP PL left | Mild radiculopathy L5 left | 1 day | complete |
| E.P., m, 72 years | id-em | L3 | neurinoma | Complete excision from dorsal | 2.8 | cmEP, ncEP | All available | Deterioration of PN and FN sensory EP left | Mild radiculopathy L4 left | 2 days | complete |
| S.R., f, 56 years | im | C0-T1 | Congenital tumor with ACM I and Syrinx | Decompression dorsal | 3.8 | csEP, cmEP, ncEP | All available | Deterioration of ncEP MN left | Numbness left arm | 1 hour | complete |
| C.Y., m, 43 years | im | L1-S2 | ependymoma | Excision complete from dorsal | 7.6 | csEP, cmEP, ssEP, nsEP, ncEP, in situ Stimulation, cont EMG, BCR | All available, but pathologic | alteration of cmEP leg right | Weakness right leg | 6 hours | complete |
| K.A., m, 36 years | im | L3-S1 | ependymoma | Complete excision from dorsal | 7.5 | cmEP, smEP, nsEP, BCR, | All available, but all pathologic | Alteration of cmEP left leg and loss of BCR right | Weakness left leg | 2 months | complete |
| D.M., m, 38 years | im | T10&11 | Lipofibroma | Partial excision from dorsal | 6 | csEP, cmEP, smEP, ssEP, nsEP, ncEP, in situ stimulation | All available, but pathologic | Alteration of cmEP TA and AH right>left | Radiculopathy L5 und S1 right > left | 6 months | Recovery to pre-existing deficits |
| F.N., f, 27 years | e | T1-T3 | Aneurysmatic cyst | Complete tumorvertebrectomy and fusion dorsal | 5 | csEP, cmEP, ncEP, nsEP, cont EMG | All available | Complete loss of all cmEP after columnotomy. | paraplegia | 4 hrs | Complete |
| F.M., f, 66 years | id-em | T12-L1 | Meningeoma recurrence | Complete tumorexcision from dorsal | 3.8 | csEP, smEP, nsEP, ncEP, in situ stimulation | All available | Deterioration of cmEP and ncEP left leg | Mild deterioration of pre-existing paraparesis | 2 weeks | Complete to pre-existing deficits |
| S.B., f, 48 years | id-em | T7&8 | Chronic leptomeningitis | Partial resection from dorsal | 3.3 | csEP, cmEP, ssEP, nsEP | All available | Deterioration of all motor and sensory EP | Mild deterioration of paraparesis | 3 weeks | complete |
| G.M., f, 54 years | id-em | C3-C5 | meningeoma | Complete resection from dorsal | 7.8 | csEP, cmEP, nsEP, ncEP | All available, but severly pathologic | Deterioration of sensory and motor EP left | Deterioration of pre-existing spasticity and ataxia | 3 months | complete |
| P.M., f, 53 years | im | C0-T2 | Congenital tumor with ACM I and Syrinx | Decompression C0-C2 | 4.5 | cmEP, ncEP | All available, but pathological | Deterioration of ncEP MN bds | Deterioration of ataxia | 3 weeks after ventriculoperitoneal shunt implantation | Recovery to pre-existent deficits |
| S.H., f, 22 years | im | C0-T4 | Congenital tumor with ACM I and Syrinx | Decompression from dorsal | 4.3 | csEP, cmEP, nsEP, ncEP | All available | Loss of ncEP MN left and alteration of ncEP TN left | Hemiataxia left | 1 month | complete |
| R.P., m, 51 years | im | C0-T12 | Congenital tumor with ACM I and Syrinx | Decompression dorsal (Shunt) | 2.8 | cmEP, ncEP | All available, but pathological | Deterioration of ncEP TN and MN left | Hemiataxia left | 3 months | complete |
| S.I., f, 80 years | im | C1-C5 | ependymoma | Complete excision from dorsal | 5.3 | csEP, cmEP, ssEP, nsEP, ncEP, cont EMG | All available, but strongly pathologic | Loss of ncEP MN and cmEP BR bilateral and cmEP ADM left | Sensomotor tetraparesis right > left | 6 months | Recovery to pre-existing deficits |
| S.E., f, 87 years | im | C5-T1 | ependymoma | Partial resection from dorsal | 5 | csEP, cmEP, smEP, ssEP, nsEP, ncEP | All available, but pathologic | Alteration of all motor EP | Mild increase of spasticity | 3 weeks | Recovery to pre-existing deficits |
| F.Pl., m, 56 years | im | C3-C5 | neurinoma | Complete resection from dorsal | 5 | csEP, cmEP, ncEP | All available, but pathologic | Loss of ncEP MN right, reduction of ncEP MN left | Increased ataxia right > left | 4 months | Recovery to pre-existing deficits |
| M.L., m, 44 years | id-em | S1-lumbosacral plexus | ganglioneuroma | Decompression and complete excision, dorsoventral | 12.3 | cmEP, ncEP, in situ stimulation | All available | Loss of BCR and BAR right, ncEP TN right | Partial sphincter palsy | 3 months | complete |
During monitoring of two patients with pre-existing mild paraparesis (chronic leptomeningitis, recurrence of meningioma at the thoracolumbar junction) the continuous monitoring predicted a worsening of the paraparesis due to the deterioration of the motor and sensory evoked potentials. The neurophysiological diagnosis has been confirmed by clinical postoperative examination, both patients recovered to the pre-existing situation after two respectively 3 weeks.
One patient, a 27 year-old female with aneurysmatic cyst at the level of T1 till T3 underwent complete gross tumor resection with vertebrectomy and dorsal fusion. The operative time was 5 h however the continuous monitoring after operation was performed in a total of 9.5 h. At the baseline all potentials have been available and the monitoring of ascending and descending pathways have been performed by application of epidural electrodes cranially and caudally to the region being operated. The surgeon performed first the cranial columnotomy and approximately after 1.5 h there was a complete loss of D-wave as well as of all other motor evoked potentials however, the neuro-cerebral potentials were present. Due to the neurophysiological findings the neurologist not only alerted but warned the surgeon of possible irreversible paraplegia, however at that moment the operation could not be stopped and as a measure to prevent irreversible damage of the spinal cord a systemic application of high dose corticosteroid was applied. In addition, as a result of a joint decision of the surgeon and neurologist, finally the application of local hypothermia of the spinal cord was applied. Unrelated to the loss of D-waves and other motor-evoked potentials, the surgeon completed the columnotomy below the level of T3 and completely removed the aneurysmatic cyst followed by vertebroplasty and dorsal fusion. The patient was monitored for another 4 h after the operation although the potentials were not elicitable and, surprisingly, 4 h after the operation the young patient recovered completely with normal follow-up without any complication and/or subjective or objective symptoms and findings.
Seven patients from the group of true positive monitored findings presented signs which indicated a postoperative onset and/or deterioration of pre-existing spasticity which could be confirmed by clinical examination. All patients recovered within 2 days and one within 4 months.
One patient with S1 lumbosacral plexus ganglioneuroma underwent decompression and gross tumor resection followed by dorsoventral fusion, (Op-time 12 h). At the baseline all potentials were available, however, during the operation the bulbocavernous reflex and the bulbo-anal reflex on the right side disappeared and postoperative partial incontinence was expected and confirmed by clinical examination and again recovered within three months completely.
In summary, the true positive monitored cases of spinal tumors with high degree of potential risk to neural structure and high technical difficulties were successfully monitored using all possible modalities being chosen according to the potential damage to the neural structure in the course of operation.
Discussion
The management of intramedullary and extramedullary spinal tumors requires a close collaboration of spine surgeons and neurologists specialised in the intraoperative neurophysiological monitoring.
From previous reports it is obvious that intraoperative SEP only is of limited value while recording only ascending sensory pathways and gives little or no information about the function of descending motor pathways. The inadequacy of SEP for monitoring of the functional integrity of spinal cord and nerve roots has been documented in several reports [6, 8, 9, 17]. The introduction of epidural electrodes for direct stimulation of spinal cord and more over the monitoring of D-wave by transcranial stimulation and recording with epidural electrodes [1, 16] was a major advancement for monitoring of the functional integrity of the spinal motor descending pathways. Epstein promoted a pioneering work in the surgical management of intradural tumors by introducing intraoperative neurophysiological monitoring for aggressive guided excision with attempted gross total resection of the tumor [2–5]. The report from the same group about the series of 100 consecutive tumor surgery procedures [7] impacted the management of spinal tumors in several centres including ours. The most important prerequisite for such a management is the close collaboration between the spine surgeon and the neurologist already at the stage of diagnosis and planning of the surgical procedure in order to prepare the appropriate modalities for the monitoring in advance. The understanding of the surgical and neurophysiological procedures by both the surgeon and the neurologist and vice versa is the decisive factor in facilitating the discussion during the operation when decision has to be made instantly in order to prevent neural damage. In our series 109 patients out of 1,017 patients who were monitored over the past 6 years have been diagnosed with spinal tumors. The distribution of 23 intramedullary, 41 intradurally and 45 epidural tumors, the clinical and histological diagnosis, the duration of operation are indirect indicators of the severity of the clinical conditions in our patient population. The decision-making process as related to the continuation and/or adaptation of the surgical procedure has been done between the surgeon and neurologist in the means of shared decision and responsibility. The shared responsibility not only results in the documentation of the 24 true positive findings with new onset of neurological deficit after the operation but also is expressed in the 82 surgical cases where true negative findings have been monitored. Particularly in this group the surgeon has been alerted whenever significant changes of the potentials occurred in order that he was capable to timely adapt his surgical manipulations to avoid permanent neurological damage. In addition the surgeon has been guided by the monitoring results allowing him the gross tumor resection (“going to the limit”) The detailed analysis of the “true negative” cases and the impact of monitoring on the surgical procedure is subject to ongoing study.
The two false positive and false negative cases were attributed only to minor or less relevant neurological deficit in comparison to the pathology being approached by the surgery. The fact that the true positive and false negative cases recovered completely or to the pre-existing neurological status, is not only the expression of the surgical skills which could be applied in the procedure but also the guidance of the intraoperative monitoring.
The one case with complete loss of motor evoked potentials after columnotomy of T1 and T3 of duration more than 3.5 h would normally result in a complete paraplegia especially while the very sensitive D-wave disappeared for the entire period of the time. We have no clear explanation for the complete recovery of the patient within 4 hours but it is possibly a result of the positive effect of the administration of systemic glucocorticosteroids and maybe the locally applied hypothermia. This case opens the discussion about neuroprotection by medications during the surgical procedure.
In summary, the multimodal intraoperative monitoring during surgery of the spinal tumor proved to be a valid and reliable method to contribute to the improvement of the surgical results allowing gross tumor resections where needed and definitely contributing to reduction or even prevention (through negative findings) of neurological damage during the surgical procedure.
Acknowledgments
Dr. Lote Medicus fund for the financial support of the development of MIOM at the Schulthess Clinic. Dave O’Riordan and Charles McCammon for helping with the manuscript. Anne Mannion Ph.D. for the critical review of the manuscript.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- 2.Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93:183–193. doi: 10.3171/jns.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 3.Cooper PR, Epstein F. Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 1985;63:492–499. doi: 10.3171/jns.1985.63.4.0492. [DOI] [PubMed] [Google Scholar]
- 4.Epstein F. Spinal cord astrocytomas of childhood. Adv Tech Stand Neurosurg. 1986;13:135–169. [PubMed] [Google Scholar]
- 5.Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993;79:204–209. doi: 10.3171/jns.1993.79.2.0204. [DOI] [PubMed] [Google Scholar]
- 6.Ginsburg HH, Shetter AG, Raudzens PA. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials. Case report. J Neurosurg. 1985;63:296–300. doi: 10.3171/jns.1985.63.2.0296. [DOI] [PubMed] [Google Scholar]
- 7.Kothbauer K, Deletis V, and Epstein F (1998) Motor evoked potential monitoring for intramedullary spinal cord tumor surgery: Correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. http://www.aans.org/journals/online_j/may98/4-5-1 [DOI] [PubMed]
- 8.Lesser RP, Raudzens P, Luders H, Nuwer MR, Goldie WD, Morris HH, 3rd, Dinner DS, Klem G, Hahn JF, Shetter AG, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22–25. doi: 10.1002/ana.410190105. [DOI] [PubMed] [Google Scholar]
- 9.Minahan RE, Sepkuty JP, Lesser RP, Sponseller PD, Kostuik JP. Anterior spinal cord injury with preserved neurogenic ‘motor’ evoked potentials. Clin Neurophysiol. 2001;112:1442–1450. doi: 10.1016/S1388-2457(01)00567-3. [DOI] [PubMed] [Google Scholar]
- 10.Quinones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, McDermott MW, Weinstein PR. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56:982–993. doi: 10.1227/01.NEU.0000156784.46143.A5. [DOI] [PubMed] [Google Scholar]
- 11.Sala F, Krzan MJ, Deletis V. Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv Syst. 2002;18:264–287. doi: 10.1007/s00381-002-0582-3. [DOI] [PubMed] [Google Scholar]
- 12.Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, Bricolo A. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–1143. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]
- 13.Shrivastava RK, Epstein FJ, Perin NI, Post KD, Jallo GI. Intramedullary spinal cord tumors in patients older than 50 years of age: management and outcome analysis. J Neurosurg Spine. 2005;2:249–255. doi: 10.3171/spi.2005.2.3.0249. [DOI] [PubMed] [Google Scholar]
- 14.Sutter M, Eggspühler A, Grob D, Jeszenszky D, Porchet F, Muller A, Dvorak J (2007) The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: a prospective study of 1017 cases. Eur Spine J (in preparation) [DOI] [PMC free article] [PubMed]
- 15.Sutter M, Eggspühler A, Muller A, Dvorak J (2007) Multimodal intraoperative monitoring: methodology. Eur Spine J (in preperation) [DOI] [PMC free article] [PubMed]
- 16.Tamaki T, Noguchi T, Takano H, Tsuji H, Nakagawa T, Imai K, Inoue S (1984) Spinal cord monitoring as a clinical utilization of the spinal evoked potential. Clin Orthop Relat Res 58–64 [PubMed]
- 17.Wiedemayer H, Sandalcioglu IE, Armbruster W, Regel J, Schaefer H, Stolke D. False negative findings in intraoperative SEP monitoring: analysis of 658 consecutive neurosurgical cases and review of published reports. J Neurol Neurosurg Psychiatry. 2004;75:280–286. [PMC free article] [PubMed] [Google Scholar]