Summary
Supramolecular chemistry has been employed to develop flexible and adaptable multivalent neoglycoconjugates for binding galectin-1 (Gal-1). Gal-1, a dimeric lectin with two galactoside-binding sites, regulates cancer progression and immune responses. Self-assembled pseudopolyrotaxanes comprised of lactoside-displaying cyclodextrin (LCD) “beads” threaded onto polyviologen “strings,” display mobile ligands as a result of cyclodextrin rotation about, and limited translation along, the polymer chain. The pseudopolyrotaxanes rapidly and efficiently precipitate Gal-1 and provide valency-corrected enhancements of up to 30-fold over native lactose and 20-fold over free LCD in a T-cell agglutination assay. A supramolecular stastical effect was observed, wherein the efficacy of Gal-1 inhibition correlates with the number of ligands connected to each other solely through mechanical and noncovalent interactions. Such flexible and adaptable self-assembled pseudopolyrotaxanes show promise for the study of multivalent interactions and targeting of therapeutically relevant lectins.
Introduction
As the static, lock-and-key view of ligand-receptor complexes gives way to more dynamic models [1], the role of flexibility and adaptability in both one-to-one and multivalent binding events has emerged as a major question in the field of molecular recognition. Multivalent interactions [2, 3] are especially prevalent in glycobiology [4–7], where high avidity interactions take place between multimeric and/or membrane-bound carbohydrate-binding proteins (lectins) and their cognate carbohydrate ligands (epitopes), despite the low affinity provided by a typical monovalent interaction between a lectin and its epitope. The fast on- and off-rates of the individual interactions and the fact that many multivalent protein-carbohydrate interactions take place at the fluid and dynamic interface of the cell surface [8], suggest that flexibility and adaptability may play important roles in these interactions.
Glycobiology, and, in a more general sense, all of biochemistry is conceptually related to the field of supramolecular chemistry, through the shared focus on molecular recognition and assembly [9, 10]. However, few of the growing class of synthetic supramolecular assemblies [3, 9–11] such as pseudorotaxanes (ring around a thread) and pseudopolyrotaxanes (many rings around a thread) and related mechanically-interlocked molecules [11–13] such as rotaxanes (ring around a dumbbell-shaped thread), catenanes (ring threaded through another ring), and polyrotaxanes (many rings on a dumbbell-shaped component) have been examined in biochemical contexts [14–18]. Recently, we [19–21] and Yui and co-workers [22–25] have used supramolecular chemistry to develop architectures for multivalent binding that are at the flexible and adaptable end of the ligand-display spectrum.
Chemists have synthesized a large variety [26–36] of neoglycoconjugates ranging from small molecules to macro- and supra-molecular entities in attempts to understand, mimic, and perturb natural multivalent lectin-carbohydrate interactions. Factors [30–33] such as rigidity, spacing, topology, and density of saccharides are known to influence the avidity of multivalent carbohydrate-displaying structures for their respective lectins. Lehn and co-workers have utilized dynamic combinatorial chemistry [34] to select trivalent neoglycoconjugates for the lectin concanavalin A. Other promising approaches that exploit multivalency for lectin binding include neoglycopolymers [4, 27, 29, 31] and dynamic self-assembled systems [26, 35, 36], such as micelles and liposomes. Carbohydrate-displaying (pseudo)polyrotaxanes [19–24] potentially combine advantages from both of these approaches for multivalent ligand presentation, including (1) the ability to span large distances, (2) easily variable ligand densities, (3) adaptability, and (4) ease of synthesis through self-assembly. Both our system [19–21] and those of Yui and co-workers [22–24] are based on the pioneering work of Harada [37] and Wenz [38] on cyclodextrin (CD)-based (pseudo)polyrotaxanes (recently reviewed in [39]). CDs are naturally occurring cyclic oligosaccharides comprised of 6, 7, or 8 glucopyranoside units. While many of the exotic structures produced by supramolecular chemists are incompatible with aqueous media, CDs are well known to encircle hydrophobic molecules in water. In many cases the encapsulated products, even large (pseudo)polyrotaxanes [39], retain water solubility. In such “beads-on-a-string” architectures, the CD rings are able to spin around the axis of the polymer chain as well as move back and forth along the polymer backbone. The extent of these movements can be varied by the choice of polymer chain [19, 24, 37–39]. If one considers a ligand attached to each CD ring, the position of this ligand is, to a large extent, independent of the position of a ligand on a neighboring ring, for while the ligands are bound via noncovalent interactions of the CD rings with the encircled polymer backbone, they are not directly covalently bound to each other. Both ligands will have considerable freedom, including up to 360° of rotation, to respond to a receptor. Using lactoside-displaying α-CD rings and a polyviologen backbone, we have recently described a self-assembled multivalent pseudopolyrotaxane [20] that was used to target galectin-1 [8, 40–44] (Gal-1), a bivalent galactoside-binding lectin.
Gal-1 is a soluble 14 kDa dimeric lectin whose natural ligands typically––but not exclusively [45]––reside on cellular membranes. Gal-1 binding mediates the organization [5, 6, 8] of cell surface glycoproteins through cross-linking of terminal or polymeric N-acetyllactosamine residues. Gal-1 is the prototypical member of the galectins [44–49], a ubiquitous family of galactoside-binding lectins that mediate cell adhesion, signaling, and death. Certain galectins, particularly Gal-1 and Gal-3, are over-expressed in a variety of cancers [43, 44, 48, 49]. In addition to promoting motility and metastasis [46], certain cancer cells are able to use Gal-1 as a self-defense mechanism [42, 43] by taking advantage of the natural function [41, 47] of Gal-1 as an apoptotic signal for T- and other cells of the immune system. Topologically, Gal-1 presents a particularly interesting challenge for targeting with synthetic multivalent ligands (recently reviewed in [44]) since it is a rigid dimer [40] with two binding sites oriented in opposite directions such that the entrance to each of the binding sites is located 6 nm apart. Since the biological targets of Gal-1 reside on the cell surface, one strategy for targeting Gal-1 is to use structures that mimic the fluidity and adaptability of cellular membranes.
In our original report [20], a pseudopolyrotaxane (Fig. 1A) composed of a polyviologen chain with, on average, 17 repeating units, over 90% threaded with lactoside-displaying CDs (LCDs), proved effective at targeting Gal-1. The pseudopolyrotaxane displays highly mobile ligands as a result of cyclodextrin rotation about, and limited translation along, the polymer chain. Multivalent enhancements of 6.7-fold and 10-fold relative to the free lactoside-CD and free lactose, respectively, were obtained for the inhibition of Gal-1 in a T-cell agglutination assay. This valency-corrected enhancement was greater than observed for lactoside-bearing trivalent glycoclusters and a fully covalent (chitosan-based) polymer tested using the same assay. Herein, we investigate these multivalent interactions between Gal-1 and lactoside-displaying supramolecular assemblies further by using T-cell agglutination and quantitative precipitation assays to evaluate pseudopolyrotaxanes with different degrees of threading, self-assembled from polyviogens of variable lengths.
Figure 1. Self-Assembled Multivalent Pseudopolyrotaxanes.
(A) Schematic representation of pseudopolyrotaxanes composed of lactoside-cyclodextrin (LCD) rings and polyviologen threads. Chemical structures of the components are also shown.
(B) Table describing the degree of threading for a series of pseudopolyrotaxane assemblies. a Dilution to 1 mM polyviologen repeating unit. TBA%, the degree of threading as measured by the Bradford assay, is defined as: 100 × [1 – (OD595 sample – OD595 background)/(OD595 free PV – OD595 background)]. The values in the table are reproduced from a full account [21] of the development of the assay. Using the first entry as an example, this pseudopolyrotaxane was self-assembled from a ratio of 5 LCDs to each PV-21 thread, hence the nomenclature [5:21]. Since the thread has, on average, 21 polyviologen repeating units, this assembly is expected to be quarter-threaded at equilibrium following self-assembly, in good agreement with the measured TBA of 23% for the stock solution (20 mM in LCD). Upon dilution equilibrium is re-established (≥2 days) with a TBA of 13% for this assembly, but this dethreading is not yet significant at 4 hrs after dilution (22% TBA).
Results and Discussion
Supramolecular Assemblies
Polyviologen AB-copolymers, comprised of alternating decamethylene (A) and positively-charged bipyridinium (B) segments, form [20, 37–39] stable, water-soluble complexes with α-CD, wherein the positive charges, associated formally with the nitrogen atoms on the bipyridinium segments of the polymer, act as electronic “speed bumps” [39, 50] which reduce the translational motion of the CD rings. In aqueous solution, the α-CD rings thread onto the polymer chain and rest predominantly on its decamethylene segments, stabilized by the hydrophobic interactions inside the cavities of the CDs. By separating the hydrophobic segments into domains flanked by “speed bumps” polyviologens can support high levels of threading at equilibrium, with the time-scale of self-assembly being relatively slow (3 – 30 days), due to the necessity of the α-CD rings passing over multiple bipyridinium segments. For example, in the self-assembly of the pseudopolyrotaxane from our earlier report [20], a mixture of 17 equivalents of LCD (20 mM) with a polyviologen containing, on average, 17 repeating units (PV-17) resulted in over 90% threading. This assembly is henceforth designated [17:17] to reflect the equivalents of ring to thread in the solution, not necessarily on polyviologen backbone: the average number of polyviologen repeating units in the backbone (i.e., 17 for PV-17). The actual degree of threading (>90%) was estimated from 1H NMR spectroscopy and TLC. It was also reported that a 50-fold dilution resulted in no change in the 1H NMR spectrum even after a week. As described elsewhere [21], there is now reason to believe that the results of this original 1H NMR dilution experiment are not general. In the course of developing a quantitative precipitation assay (vide infra), we had the occasion to subject these pseudopolyrotaxanes to the Bradford assay [51] (Bio-Rad), a well-known analytical method for quantifying protein concentrations based on a blue-shift in the absorption spectra of the dye, Coomassie Blue. This assay resulted in several surprising discoveries [21]: (1) free polyviologens are responsive to the Bradford assay, which is usually highly selective for proteins; (2) for pseudopolyrotaxanes derived from polyviologens, the response of the Bradford assay was dependent on, and thus indicative of, the degree of threading; and using this simple colometric assay as a reporter, (3) dethreading was observed for diluted solutions of the pseudopolyrotaxanes upon standing. While these pseudopolyrotaxanes do not appear to be as thermodynamically stable to dilution as previously supposed, they are kinetically stable on the time-scale (15 minutes) of the T-cell agglutination assays. As a result of the electronic speed bumps provided by the viologen units, it can take up to two days to reach equilibrium upon dilution; initial dethreading is observed, however, on the time-scale (3 hours) of the precipitation experiments presented herein.
The polyviologens PV-8 and PV-21 were synthesized according to literature precedent [50]. The number average molecular weights (Mn = 3650 and 9581), which were determined (end-group analysis) by 1H NMR spectroscopy, correspond to an average of 8 and 21 repeating AB units, respectively. PV-21 is somewhat longer than the previously reported PV-17 while PV-8 is considerably shorter. Seven solutions (Figure 1B) were prepared with 20 mM LCD in H2O and different amounts of either PV-8 or PV-21. With an equal number of rings to repeating units solutions [21:21] and [8:8] are expected to contain nearly fully threaded pseudopolyrotaxanes and few free LCDs. With (roughly) half as many rings as repeating units, solutions [10:21] and [4:8] are expected to be nearly half-threaded, while [5:21] and [2:8] with (roughly) a quarter of rings to repeating units, are expected to be quarter-threaded. By contrast, solution [42:21] contains twice as many rings as repeating units, so that it should contain a (very nearly) fully threaded pseudopolyrotaxane and a full equivalent of free LCD. Results from the Bradford assay are shown in Figure 1B as TBA (Threading by Bradford Assay) percentages [21] for these solutions at the concentration (20 mM LCD) used for self-assembly. Note that the TBA percentages correlate extremely well with our expectations of threading by the PV-21-derived pseudopolyrotaxanes and also report––apparently over-estimating––the degree of threading for PV-8 derived pseudopolyrotaxanes. The aqueous solutions of self-assembled pseudopolyrotaxanes (20 mM LCD) were used as stock solutions for the T-cell agglutination and quantitative precipitation experiments, which were performed immediately following dilution unless otherwise noted. Diluted solutions of the pseudopolyrotaxanes experienced dethreading upon standing, as observed by lower TBA percentages (Figure 1B, dilution to 1 mM polyviologen repeating unit), which correlated with decreased activity in functional assays (vide infra). Note that assembly [21:21], for which this concentration is a 20-fold dilution in both LCD and PV-21, goes from 87 to 52% TBA, reaching equilibrium in two days, while assembly [42:21], which contains the full extra equivalent of LCD, is much more resistant to dethreading. While some dethreading is observed for all pseudopolyrotaxanes after two days, [21:21], [10:21], [8:8], and [4:8] show noticeable dethreading after only 4 hours.
Quantitative Precipitation Assay
It is well established that multivalent lectins and carbohydrates can induce each other to precipitate [5, 6, 30, 31]. If it is possible to distinguish between the lectin and carbohydrate portion of the precipitate, quantitative precipitations [52] can be performed to gain such information as the ratio of lectin to carbohydrate in the precipitate. The Bradford assay was used as a protein-specific reporter, while UV spectroscopy provides the total amount of both protein and polymer in the solution and in the precipitate (see Experimental Procedures for details). The responsiveness of the pseudopolyrotaxanes themselves to the Bradford assay [21] limits our ability to quantify precipitation results by this method to those concentrations and degrees of threading that do not yield a significant Bradford response. However, at concentrations relevant to the precipitation assay, many of the assemblies and in particular the highly effective [21:21] and [42:21] do not yield a Bradford response above background, so in these cases, the assay can be used to report solely on protein concentration in the presence of pseudopolyrotaxane.
Neither the pseudopolyrotaxanes, their components, nor recombinant human Gal-1 [53] precipitate on their own, at least during the time-scale of these experiments. The individual pseudopolyrotaxane components, LCD, PV-8, and PV-21, do not induce Gal-1 to precipitate. With the pseudopolyrotaxanes, precipitation (Figure 2a) is rapid as determined visually or by light scattering (Supplemental Data). A white precipitate is clearly visible within the first minute of mixing for concentrations such as 4:1 polyviologen repeating unit of assembly [21:21] to Gal-1 (50 μM). As it occurs readily in both polypropylene Eppendorf tubes and polysyrene 96-well plates, precipitation was not dependent on a particular type of surface. After mixing solutions were left for at least 3 hours before separating the solution from the precipitate (all precipitations are likely complete at around 2 hours, based on light scattering). The precipitate was then dissolved in 0.5 M lactose, which was also added to the separated solution––otherwise additional aggregation/precipitation phenomena occur as the equilibrium is re-established after the removal of the precipitate––prior to analysis by the Bradford assay and UV spectroscopy.
Figure 2. Quantitative Precipitation Assay of Pseudopolyrotaxane [21:21] with Gal-1.

(A) Chart describing the concentrations of Gal-1 and pseudopolyrotaxane [21:21] in the precipitates obtained from two separate titrations. On the left, increasing concentrations of pseudopolyrotaxane [21:21] as well as its components LCD and PV-21 were mixed with a fixed concentration (50 μM) of Gal-1. On the right increasing concentrations of Gal-1 were mixed with a fixed concentration (50 μM) of pseudopolyrotaxane [21:21]. The concentrations of pseudopolyrotaxane [21:21] indicated are per polyviologen repeating unit.
(B) Chart comparing the ratio of Gal-1 and pseudopolyrotaxane [21:21] in the precipitates obtained from both titrations.
(C) Idealized model of precipitate highlighting the 2:1 ratio of polyviologen repeating units to Gal-1 found in the precipitate across a 10-fold range of starting pseudopolyrotaxane [21:21] concentrations.
The pseudopolyrotaxanes efficiently precipitate Gal-1 (Figure 2a), with assembly [21:21] inducing over 80% precipitation at a 4:1 starting ratio of the polyviologen repeating unit to Gal-1, which results in a 2:1 ratio of polyviologen repeating unit to Gal-1 in the precipitate. This ratio (Figure 2b) is relatively constant over a 10-fold titration range of assembly [21:21] to Gal-1, and represents a cross-linking Gal-1 occupying every other potential binding site in the precipitated pseudopolyrotaxane-lectin aggregates. See Figure 2c for an idealized model of the precipitate. This ratio of polyviologen repeating unit to Gal-1 in the precipitate can be driven to nearly 1:1 with an excess of Gal-1 relative to the polyviologen repeating units (Figure 2b). Note this ratio is not expected to hit exactly 1:1, given that not every repeating unit of the polyviologen backbone will be occupied by an LCD. Normalized to the concentration of polyviologen repeating units, assemblies [8:8] (Figure 3a) [10:21] and [5:21] were all less efficient at precipitating Gal-1 than assembly [21:21] (Figure 3b). However, assembly [42:21], which contains an extra LCD for every polyviologen repeating unit, was somewhat more efficient than assembly [21:21] (Fig. 3b). This result was a surprising one, since free LCDs were initially expected to detract from precipitation efficiency. The precipitates from assembly [42:21] displayed Gal-1 to polyviologen repeating unit ratios close to 1:1, even at excess polyviologen repeating unit to Gal-1 ratios. This observation reflects the increased likelihood of each polyviologen repeating unit to be threaded in assembly [42:21], especially considering its resistance to dethreading, compared with the propensity of pseudopolyrotaxane [21:21] to dethread over relatively short time-scales (Figure 1b). This increased propensity to be threaded most likely accounts for the increased precipitation efficiency of pseudopolyrotaxane [42:21], over-coming, or perhaps even aided by, the equivalent of free LCD. While a large excess of free lactoside inhibits and reverses precipitation, one equivalent could aid precipitation by breaking up unproductive aggregates/chelates in favor of those more prone to precipitate.
Figure 3. Quantitative Precipitation Assay of Pseudopolyrotaxanes with Gal-1.

(A) Chart describing the concentrations of Gal-1 and pseudopolyrotaxane [8:8] in the precipitates obtained from two separate titrations. On the left, increasing concentrations of pseudopolyrotaxane [8:8] were mixed with a fixed concentration (50 μM) of Gal-1. On the right increasing concentrations of Gal-1 were mixed with a fixed concentration (50 μM) of pseudopolyrotaxane [8:8]. The concentrations of pseudopolyrotaxane [8:8] indicated are per polyviologen repeating unit.
(B) Chart describing concentrations of Gal-1 and PV-21-based pseudopolyrotaxanes in the precipitates obtained from two separate titrations. On the left, increasing concentrations of pseudopolyrotaxanes [42:21], [21:21], [10:21], and [5:21] were mixed with a fixed concentration (50 μM) of Gal-1. On the right increasing concentrations of Gal-1 were mixed with a fixed concentration (50 μM) of each pseudopolyrotaxane. The concentrations of pseudopolyrotaxanes indicated are per polyviologen repeating unit.
Precipitation is occurring on a time-scale that is competitive with at least the beginnings of dethreading. Dethreading prior to exposure to Gal-1 can limit the effectiveness of precipitation, as was observed when diluted solutions of assembly [21:21] were allowed to stand for 3 hours before beginning a precipitation experiment, which resulted in a small but noticeable decrease in the amount of Gal-1 precipitated––70% with a 4:1 ratio of polyviologen repeating unit to Gal-1 for [21:21], data not shown. Once exposed to Gal-1, cross-linking almost certainly alters the dethreading kinetics and thermodynamics.
T-Cell Agglutination Assay
A straightforward assay [20] based on the ability of Gal-1 to aggregate T-cells was employed to evaluate the psuedopolyrotaxanes. Treatment of CEM cells––cultured human T-leukemia cells––with 10 μM recombinant human Gal-1 [53] for 5 min at 37° C results in large aggregates that resemble tumor emboli, while pre-treating Gal-1 with lactosides for 10 min at room temperature results in either similar aggregates, a mixed state of aggregated and dispersed cells, or mostly dispersed cells (Figure 4 panels g, d, and a, respectively, for example). The lowest value of lactoside where the mixed state is observed is defined as the minimum inhibitory concentration (MIC). Inhibition has been shown [20] to be lactoside-dependent, as free polyviologens or polyviologens threaded with native α-CD are not inhibitors. The PV-21 and PV-8-based assemblies, always freshly diluted into PBS buffer from the H2O stock solution, were subjected to the agglutination assay (Figures 4–6).
Figure 4. T-cell Agglutination Assay.
Light microscopy (10x) images of titrations of pseudopolyrotaxanes [42:21], [21:21], and [5:21] in the presence of CEM cells and 10 μM Gal-1. Valency-corrected lactoside concentrations are shown at the left. At 600 μM in lactoside cells are: (a) dispersed for [42:21], (b) dispersed for [21:21], (c) in the mixed state for [5:21]. At 300 μM in lactoside cells are: (d) in the mixed state for [42:21], (e) in the mixed state for [21:21], (f) in the mixed state for [5:21]. At 100 μM in lactoside cells are: (g) aggregated for [42:21], (h) in the mixed state for [21:21], (i) in the mixed state for [5:21].
Figure 6. Supramolecular Statistical Effect: A Dependence on the Number of Noncovalently Connected Ligands.
(A) Table highlighting the agglutination assay results––lowest values of inhibitor necessary to obtain the dispersed and mixed states on a per-polymer basis––as a function of the number of non-covalently connected ligands (average (maximum) number of LCDs/polymer).
(B) Graph of the log average (maximum) number of LCDs/polymer vs. the negative log of the lowest polymer concentration necessary to obtain the mixed and dispersed states. The linear fits reveal a better correlation for the dispersed state. The large outlier for the mixed state is assembly [5:21].
At an intermediate per-lactoside concentration of 300 μM (panels d-f in Figure 4), assemblies [4:21], [21:21], and [42:21] have all yielded the mixed state. At 100 μM (panels g–i in Figure 4), which is the MIC of assembly [21:21] (Figure 5a), the inhibitory affect of assembly [42:21], which contains the extra equivalent of LCD and thus might be expected to be half as effective as assembly [21:21] on a valency-corrected basis, has indeed been lost. By contrast, the quarter-threaded pseudopolyrotaxane [5:21] is still inhibitory. In fact, the pseudopolyrotaxane [5:21] displays a valency-corrected MIC of 50 μM (Figure 5a), a 30-fold enhancement over lactose, 20-fold over free LCD, and 2-fold better than the nearly fully threaded assembly [21:21], despite having only one quarter the number of available lactoside ligands. The roughly half-threaded assembly [10:21] yields the same MIC (100 μM as the nearly fully threaded assembly [21:21], confirming that, on a per-lactoside basis, it is not necessary to maximize the number of LCDs/thread to attain the mixed state at a low concentration. However, the lower-threaded assemblies [5:21] and [10:21] attain the dispersed state only at higher concentrations (750 μM, valency-corrected) than the more fully threaded assemblies. As portrayed in Figure 4 (panels a–c), [21:21] and [42:21] have attained the dispersed state at 600 μM (valency-corrected), while [5:21] remains in the mixed state at this concentration. The PV-8-based assemblies also show that the more fully-threaded systems attain the dispersed state more readily on a valency-corrected basis, whereas the valency-corrected MIC for each of these assemblies is the same (200 μM) This result represents an enhancement of 7.5-fold vs. lactose and 5-fold vs. LCD, i.e., significant gains, at least for the quarter-threaded assembly [2:8], considering there are only two lactoside ligands (on average) connected in this pseudopolyrotaxane.
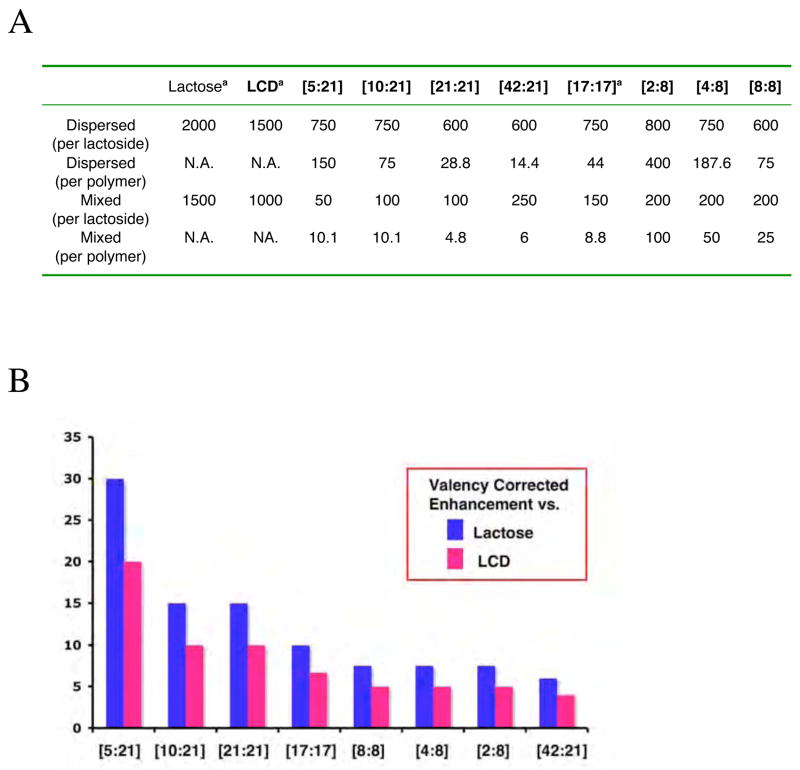
Figure 5. Summary of Agglutination Assays.
(A) Table relating the lowest value of inhibitor necessary to obtain the dispersed and mixed states (MIC) on both a per-lactoside and per-polymer basis. a Values from reference [20]. All titrations were performed at least in triplicate.
(B) Results of the agglutination assay presented as a chart of valency corrected enhancements (per-lactoside MIC normalized to MIC for free lactose (blue) or free LCD (magenta).
The multivalent enhancements (valency-corrected MIC) for the full range of pseudopolyrotaxanes tested vs. MIC for free lactose and LCD are shown in Figure 5b. The quarter-threaded 21-mer [5:21] is the best performer, while assembly [42:21], where half of the LCDs are not on located a polymer backbone, is the worst; but it still generates a 4-fold enhancement over free LCD or a 6-fold one over lactose. The nearly fully threaded [21:21] and half-threaded [10:21] gave the same enhancement (10-fold vs. LCD, 15-fold vs. lactose); thus, from the “perspective” of lactoside ligand, it does not matter whether the polyviologen backbone is half or nearly fully threaded, although it is more effective to be one-quarter threaded. Indeed, with its valency-corrected enhancement of 20-fold over the monovalent ligand (LCD), assembly [5:21] has the highest valency-corrected enhancement of which we are aware, for inhibition of Gal-1 in a cell-based assay. Gabius and co-workers have achieved much greater enhancements for Gal-1 binding as measured in solid-phase competition assays using starburst glycodendrimers [54] and wedge-shaped glycodendrons [55], synthesized by Roy and coworkers and Pieters and co-workers, respectively. At least for the glycodendrons [55], the best efficacy in a cell-based assay was on the same order of magnitude (14.5-fold over monovalent lactoside) as seen here for the potent pseudopolyrotaxanes.
There is an interesting progression observed for the nearly fully threaded pseudopolyrotaxanes [21:21], [17:17], and [8:8] correlating average polyviologen length with multivalent enhancement (10-, 6.7-, and 5-fold vs. LCD, respectively), i.e., from the lactoside ligand “perspective” it is more effective to be on a longer polymer thread, which allows for more noncovalently connected ligands and greater total bridgeable distances between the lactosides. As noted, all three of the PV-8-based assemblies gave the same multivalent enhancement of 7.5-fold vs. lactose and 5-fold vs. LCD. The PV-8-based assemblies differentiate themselves when we consider the polymer backbone (polyviologen) concentration at the MIC, which ranges from 25 μM for assembly [8:8] to 100 μM for assembly [2:8]. For the PV-21-based assemblies as well, from the “perspective” of the polyviologen backbone, the most effective assembly is the nearly fully threaded [21:21] with an MIC of 4.8 μM on a per-polymer basis. This value is half of the Gal-1 concentration (10 μM) used in these experiments, and represents an advantage of 357-fold over lactose and 208-fold over LCD on a multivalent supramolecular-complex to monovalent ligand basis.
A comparison of the polymer concentrations at the lowest values of the mixed and dispersed states for all pseudopolyrotaxanes tested reveals a general trend toward increasing efficacy with increased threading. Furthermore, by re-organizing the data in Figure 5a to Figure 6a to highlight the number of ligands (LCDs) that may be noncovalently connected to each other in each assembly, regardless of the length of the polyviologen backbone, a clear progression emerges in the values relating the number of potentially connected ligands (average maximum number of LCDs/polymer) to the polymer concentration necessary to attain the dispersed state. Indeed, expressed graphically (Figure 6b), the negative log of the polymer concentration vs. the log of the average maximum number of LCDs/polymer yields a reasonable linear fit (R2 = 0.965), indicating that the ability to attain the dispersed state is described reasonably well by this one factor alone. The same trend exists for the mixed state, but the correlation is much worse (R2 = 0.87), an observation which indicates that other factors, presumably including polymer length and ligand spacing, also strongly affect the ability to attain the mixed state. Additionally, the values for the monovalent ligand LCD, shown at 0 on the graph (log of 1), fall directly on the line for the dispersed state, and far off the line for the mixed state. By definition, the dispersed state must be at or near saturation of Gal-1 binding sites by the pseudopolyrotaxanes, as there is not enough free Gal-1 to cause substantial aggregation of the T-cells. It follows that, on a hypothetical binding curve between Gal-1 and the pseudopolyrotaxanes, the mixed state is a lower point on the curve, perhaps near the hypothetical IC50. While it is notoriously problematic [56] to relate the results of agglutination assays directly to thermodynamic binding parameters, it is not surprising that more subtle factors would be necessary to account for binding near the hypothetical IC50 than near saturation. Alternatively, the different dependence on the number of connected ligands could imply a change in the mechanism(s) of binding with increasing pseudopolyrotaxane concentration upon going from the mixed to dispersed states.
A dependence on the number of connected ligands is a hallmark of the statistical effect in multivalent binding [2–7, 30, 31, 52, 56]. Here, the ligands are not connected directly by traditional covalent bonds, but are mechanically connected via the encircled polymer backbone. Hence, we observe a supramolecular statistical effect for these pseudopolyrotaxanes inhibiting gal-1-mediated agglutination of the T-cells, particularly at the higher pseudopolyrotaxane concentrations necessary to obtain the dispersed state. The observation of a supramolecular statistical effect does not, in-and-of-itself, define a mechanism of binding, however, it is consistent with the simplest possible mechanism: one-to-one binding between Gal-1 and a single pseudopolyrotaxane chain, where only one of the lactoside binding sites is occupied at a time. Another possible mechanism of binding is chelation, whereby a single pseudopolyrotaxane chain provides lactosides to both Gal-1 binding sites. Aggregation, which in this context we explicitly define as cross-linking of different pseudopolyrotaxane chains by one or more Gal-1 dimers is also likely to be factor. While the thermodynamic driving forces behind protein-carbohydrate aggregates are still not fully understood, the formation of such aggregates has been suggested [56] to be a major factor in agglutination experiments. Furthermore, Gal-1 is an evolutionarily adapted cross-linker [4–6, 40], with a topology that would bias aggregation over chelation. The tendency of Gal-1 and the pseudopolyrotaxanes to aggregate is clear from the light scattering and precipitation experiments, albeit at different concentrations and ratios than are present in the agglutination assay.
It would be particularly interesting to know whether the pseudopolyrotaxanes chelate Gal-1. Chelation is often associated [2–7, 28, 30, 31, 33] with considerably greater multivalent enhancements than are obtained by the statistical effect alone. A traditional view [4, 7, 56] holds that chelation would be disfavored for a flexible system that will freeze out considerable entropy upon chelation, especially given the large distance between, and orientation of, lactoside binding sites in Gal-1. However, the incompatibility of chelation with flexible systems and long distances between the potentially bridging ligands has been questioned [3, 57, 58] on experimental and theoretical grounds. A definitive answer on the ability of the pseudopolyrotaxanes to chelate Gal-1 and the relative importance of this interaction to the overall binding energy will require more a direct readout for thermodynamic binding parameters. Nevertheless, deviations from the dependence on just the number connected ligands may be reporting on some degree of chelation, as certain combinations of other factors, such ligand spacing and polymer length, should favor chelation. Considerations of bond-lengths and angles in the pseudopolyrotaxanes suggest that chelation could occur for LCDs that are a minimum of 4 polyviologen repeating units apart. This spacing is intriguing as it is reasonable that the LCDs of assembly [5:21] would be, on average, 4 repeating units apart. This pseudopolyrotaxane is both the most potent on a valency-corrected basis, and the largest outlier from the trend of simple dependence on the number of connected ligands for the mixed state (Figure 6). Assembly [5:21] also maintains the mixed state over a larger concentration range than the more fully threaded pseudopolyrotaxanes (Figures 4 and 5a). Perhaps this range is expanded by access to binding modes, particularly chelation, that are less favored for the other pseudopolyrotaxanes. In comparison with aggregation, for example, it is interesting to consider how poorly pseudopolyrotaxane [5:21] performed in the quantitative precipitation assay (Figure 3b). Overall it is likely that the flexible and adaptable pseudopolyrotaxanes exhibit a variety of binding modes with dimeric Gal-1, and that the distribution among these binding modes changes with the degree of threading, which itself is a dynamic quantity.
Conclusions
Biology is replete with examples of individual proteins self-assembling into superstructures wherein the complexes gain or enhance functions with respect to those of the individual components [9, 10]. Here, supramolecular species formed from over 20 ([21:21] and [42:21]) or as few as 3 ([2:8]) self-assembling components have been used successfully precipitate the bivalent lectin Gal-1 and to inhibit Gal-1 in T-cell agglutination assays. These self-assembled pseudopolyrotaxanes are an example of the kind of flexible and adaptable multivalent ligand display that can be afforded through supramolecular chemistry. The lactoside ligands are able to rotate freely and independently around the polymer backbone, while the CDs can translate within the confines of the decamethylene units, which have a length that is twice the depth of a CD cavity. Not only do the lactoside ligands have the ability to “fine tune” their positions without enthalpic penalties having to be paid on account of inducing strain, but the unbound ligands may also reside away from the bulky protein in order to reduce steric clashes. This flexibility and adaptability may contribute to the speed, efficacy, and large amount of Gal-1 captured by the pseudopolyrotaxanes in the precipitations, as well as benefiting the supramolecular statistical effect and providing efficacy in the agglutination assay. These flexible and adaptable multivalent supramolecular complexes show promise both for the study of protein-carbohydrate interactions and the exploitation of multivalency for targeting therapeutically relevant lectins.
Significance
A key question across many fields of molecular recognition––and, in particular, glycobiology––is the degree to which flexibility and adaptability contribute to multivalent binding. Herein, we have described the interactions of a series of self-assembled multivalent pseudopolyrotaxanes, comprised of lactoside-displaying cyclodextrin (LCD) “beads” threaded onto polyviologen “strings,” with the bivalent lectin, galectin-1 (Gal-1). These supramolecular assemblies display highly flexible and adaptable ligands as a result of rotation of the cyclodextrin torus about, and limited translation along, the polymer chain. Seven assemblies with different ratios of “beads” threading two different length polyviologen “strings” have been prepared. The pseudopolyrotaxanes rapidly and efficiently precipitate Gal-1, with over 80% precipitation of Gal-1 observed. The ratio of Gal-1 to polyviologen repeating unit in the precipitate can approach 1:1, i.e., nearly every potential binding site is occupied by a cross-linking Gal-1 in the precipitated pseudopolyrotaxanelectin aggregates. A supramolecular stastical effect was observed in a T-cell agglutination assay, wherein the efficacy of Gal-1 inhibition correlates with the number of ligands connected to each other solely through mechanical and noncovalent interactions. Also in this assay, valency-corrected enhancements of up to 30-fold over native lactose and 20-fold over free LCD, as well as per-polymer enhancements of up to 357- and 208-fold over native lactose and free LCD, respectively, were obtained. These results show that synthetic supramolecular complexes are useful tools for the study of multivalent protein-carbohydrate interactions––and more broadly––suggest that the conceptually related, but in practice often disparate, fields of biochemistry and supramolecular chemistry have much to offer each other.
Experimental Procedures
General
LCD [19], assembly [17:17] (pseudopolyrotaxane 3 in reference [20]), and details of the agglutination assay [20] with CEM cells (subclone of ATCC no. CCL-119) and recombinant human Gal-1 [53] have been previously described. Polyviologens PV-8 and PV-21 were synthesized according to literature precedent [20, 50], for details and characterization see Supplemental Data. The use of the Bradford assay to monitor the degree of threading for these pseudopolyrotaxanes has been described in detail separately [21].
Pseudopolyrotaxanes
Stock solutions (20 mM LCD in H2O) of assemblies [5:21], [10:21], [21:21], and [42:21] were prepared in 1.5 mL Eppendorf tubes by dissolving LCD (10 μmol) and varying quantities of PV-21 (42, 21, 10, and 5 μmol, respectively) in 500 μL H2O. Stock solutions (20 mM LCD in H2O) of assemblies [2:8], [4:8], and [8:8] were prepared in 1.5 mL Eppendorf tubes by dissolving LCD (10 μmol) and varying quantities of PV-8 (40, 20, and 10 μmol, respectively) in 500 μL H2O. The pseudopolyrotaxanes self-assembled upon standing (1 month) prior to the initiation of biochemical experiments. The degree of threading in these assemblies was monitored using the Bradford assay [21].
Quantitative Precipitation
Precipitation reactions were conducted in 1.5 mL Eppendorf tubes and initiated by mixing human recombinant Gal-1 [53] with a pseudopolyrotaxane assembly in the molar ratios described in Figures 2–3, in total volumes of 150 μL PBS buffered solution. Following initial mixing, the tubes were allowed to sit for 3 hours and then centrifuged for 10 minutes at 14,000 rpm. The 150 μL solutions were then transferred to new 1.5 mL Eppendorf tubes (henceforth S-tubes), and 150 μL lactose solution (0.5 M lactose in PBS) was added to both sets of tubes, which were then vortexed (≥30 sec each for the precipitate-containing original tubes, henceforth P-tubes). Aliquots (2 × 10 μL, obtained from dilutions if necessary) were taken from both sets of tubes and analyzed by the Bradford assay [21], using the appropriate background (0.5 M lactose in PBS for P-tubes) and (0.25 M lactose in PBS for S-tubes). Aliquots (50 μL) were taken directly from the S-tubes for analysis by UV-Vis spectroscopy at OD263 using a microcuvette compared to a background of 0.25 M lactose in PBS. Aliquots (25 μL) from the P-tubes were added to 25 μL PBS and analyzed by UV-Vis spectroscopy at OD263 using a microcuvette compared to a background of 0.25 M lactose in PBS.
Control experiments demonstrated that at the concentrations tested, unless otherwise noted, the pseudopolyrotaxanes do not yield a Bradford response above background. Thus, the Bradford response in these samples was attributed solely to Gal-1, and was converted to a molar concentration by comparison with an independently prepared standard curve for Gal-1. Good correlations were obtained for the total measured amounts of Gal-1 from the precipitate and remaining solution compared to the starting amount of Gal-1. While the Bradford assay was used to specify for Gal-1, UV-Vis spectroscopy provided a measure of the total concentration of both Gal-1 and pseudopolyrotaxane in the samples. The concentration of both Gal-1 and the pseudopolyrotaxanes vary linearly with absorbance at 263 nm. The molar absorptivities (ε263) were obtained independently for Gal-1 and each pseudopolyrotaxane. The concentration of Gal-1 in each sample as determined by the Bradford assay was converted to an OD263 value that was subtracted from the experimentally measured OD263 for each sample. The remaining absorbance was assigned to pseudopolyrotaxane in the sample, and converted to a concentration using the ε263 for that pseudopolyrotaxane. Using this method, good correlations were obtained for the total measured amounts of pseudopolyrotaxane from the precipitate and remaining solution compared to the starting amount of pseudopolyrotaxane. Overall, the amounts of Gal-1 and pseudopolyrotaxane measured independently from the precipitate and the remaining solution agreed well with the total initial concentrations. The results obtained from analyzing the precipitate are presented in Figures 2–3. Each independent titration was performed in duplicate, and three independent titrations were performed for each assembly described.
Supplementary Material
Detailed synthetic procedures and characterization of polyviologens PV-8 and PV-21 and an example of a light scattering experiment (Figure S1) are available at…
Acknowledgments
This work was supported by the National Institutes of Health (GM63281) and the National Science Foundation (CHE-9974928). J.M.B. is grateful to the National Institutes of Health for the award of a postdoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cavasotto CN, Kovacs JA, Abagyan RA. Representing receptor flexibility in ligand docking through relevant normal modes. J Am Chem Soc. 2005;127:9632–9640. doi: 10.1021/ja042260c. [DOI] [PubMed] [Google Scholar]
- 2.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem, Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Mulder A, Huskens J, Reinhoudt DN. Multivalency in supramolecular chemistry and nanofabrication. Org Biomol Chem. 2004;2:3409–3424. doi: 10.1039/b413971b. [DOI] [PubMed] [Google Scholar]
- 4.Lee RT, Lee YC. Affinity enhancement by multivalent lectin-carbohydrate interaction. Glycoconjugate J. 2000;17:543–551. doi: 10.1023/a:1011070425430. [DOI] [PubMed] [Google Scholar]
- 5.Sacchettini JC, Baum LG, Brewer CF. Multivalent protein-carbohydrate interactions. A new paradigm for supramolecular assembly and signal transduction. Biochemistry. 2001;40:3009–3015. doi: 10.1021/bi002544j. [DOI] [PubMed] [Google Scholar]
- 6.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12:616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 7.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 8.Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- 9.Philp D, Stoddart JF. Self-assembly in natural and unnatural systems. Angew Chem, Int Ed Engl. 1996;35:1155–1196. [Google Scholar]
- 10.Fiammengo R, Crego-Calama M, Reinhoudt DN. Synthetic self-assembled models with biomimetic functions. Curr Opin Chem Biol. 2002;5:660–673. doi: 10.1016/s1367-5931(01)00263-0. [DOI] [PubMed] [Google Scholar]
- 11.Balzani V, Credi A, Raymo FM, Stoddart JF. Artificial molecular machines. Angew Chem, Int Ed. 2000;40:1216–1221. doi: 10.1002/1521-3773(20001002)39:19<3348::aid-anie3348>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Nepogodiev SA, Stoddart JF. Cyclodextrin-based catenanes and rotaxanes. Chem Rev. 1998;98:1959–1976. doi: 10.1021/cr970049w. [DOI] [PubMed] [Google Scholar]
- 13.Raymo FM, Stoddart JF. Interlocked macromolecules. Chem Rev. 1999;99:1643–1663. doi: 10.1021/cr970081q. [DOI] [PubMed] [Google Scholar]
- 14.Dvornikovs V, House BE, Kaetzel M, Dedman JR, Smithrud DB. Host-[2]rotaxanes as cellular transport agents. J Am Chem Soc. 2003;125:8290–8301. doi: 10.1021/ja034918c. [DOI] [PubMed] [Google Scholar]
- 15.Katz E, Sheeney H-I, Willner I. Electrical contacting of glucose oxidase in a redox-active rotaxane configuration. Angew Chem, Int Ed. 2004;43:3292–3300. doi: 10.1002/anie.200353455. [DOI] [PubMed] [Google Scholar]
- 16.Lam RTS, Belenguer A, Roberts SL, Naumann C, Jarrosson T, Otto S, Sanders JKM. Amplification of acetylcholine-binding catenanes from dynamic combinatorial libraries. Science. 2005;308:667–669. doi: 10.1126/science.1109999. [DOI] [PubMed] [Google Scholar]
- 17.Arunkumar EE, Forbes CC, Noll BC, Smith BD. Squaraine-derived rotaxanes: sterically protected fluorescent dyes. J Am Chem Soc. 2005;127:3288–3289. doi: 10.1021/ja042404n. [DOI] [PubMed] [Google Scholar]
- 18.Shuai X, Merdan T, Unger F, Kissel T. Supramolecular gene delivery vectors showing enhanced transgene expression and good biocompatibility. Bioconjugate Chem. 2005;16:322–329. doi: 10.1021/bc0498471. [DOI] [PubMed] [Google Scholar]
- 19.Nelson A, Stoddart JF. Dynamic multivalent lactosides displayed on cyclodextrin beads dangling from polymer strings. Org Lett. 2003;5:3783–3786. doi: 10.1021/ol0353464. [DOI] [PubMed] [Google Scholar]
- 20.Nelson A, Belitsky JM, Vidal S, Joiner CS, Baum LG, Stoddart JF. A self-assembled multivalent pseudopolyrotaxane for binding galectin-1. J Am Chem Soc. 2004;126:11914–11922. doi: 10.1021/ja0491073. [DOI] [PubMed] [Google Scholar]
- 21.Belitsky JM, Nelson A, Stoddart JF. Monitoring cyclodextrin-polyviologen pseudopolyrotaxanes with the Bradford assay. Org Biomol Chem. 2006;4:250–256. doi: 10.1039/b509576j. [DOI] [PubMed] [Google Scholar]
- 22.Ooya T, Eguchi M, Yui N. Supramolecular design for multivalent interaction: maltose mobility along polyrotaxane enhanced binding with concanavalin A. J Am Chem Soc. 2003;125:13016–13017. doi: 10.1021/ja034583z. [DOI] [PubMed] [Google Scholar]
- 23.Ooya T, Utsunomiya H, Eguchi M, Yui N. Rapid binding of concanavalin A and maltose-polyrotaxane conjugates due to mobile motion of α-cyclodextrins threaded onto a poly(ethylene glycol) Bioconjugate Chem. 2005;16:62–69. doi: 10.1021/bc049809h. [DOI] [PubMed] [Google Scholar]
- 24.Yui N, Ooya T. Molecular mobility of interlocked structures exploiting new functions of advanced biomaterials. Chem Eur J. 2006;12:6730–6737. doi: 10.1002/chem.200600370. [DOI] [PubMed] [Google Scholar]
- 25.Choi HS, Takahashi A, Ooya T, Yui N. Molecular-recognition and binding properties of cyclodextrin-conjugated polyrotaxanes. ChemPhysChem. 2006;7:1668–1670. doi: 10.1002/cphc.200600147. [DOI] [PubMed] [Google Scholar]
- 26.Kingery-Wood JE, Williams KW, Sigal GB, Whitesides GM. The agglutination of erythrocytes by influenza virus is strongly inhibited by liposomes incorporating an analog of sialyl gangliosides. J Am Chem Soc. 1992;114:7303–7305. [Google Scholar]
- 27.Bovin NV. Polyacrylamide-based glycoconjugates as tools in glycobiology. Glycoconjugate J. 1998;15:431–446. doi: 10.1023/a:1006963717646. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Merritt EA, Ahn M, Roach C, Hou Z, Verlinde CLMJ, Hol WGJ, Fan E. Solution and crystallographic studies of branched multivalent ligands that inhibit the receptor-binding of cholera toxin. J Am Chem Soc. 2002;124:12991–12998. doi: 10.1021/ja027584k. [DOI] [PubMed] [Google Scholar]
- 29.Arranz-Plaza E, Tracy AS, Siriwardena A, Pierce JM, Boons GJ. High-avidity, low-affinity multivalent interactions and the block to polyspermy in Xenopus laevis. J Am Chem Soc. 2002;124:13035–13046. doi: 10.1021/ja020536f. [DOI] [PubMed] [Google Scholar]
- 30.Woller EK, Walter ED, Morgon JR, Singel DJ, Cloninger MJ. Altering the strength of lectin binding interactions and controlling the amount of lectin clustering using mannose/hydroxyl-functionalized dendrimers. J Am Chem Soc. 2003;125:8820–8826. doi: 10.1021/ja0352496. [DOI] [PubMed] [Google Scholar]
- 31.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor–ligand binding mechanisms with multivalent ligand architecture. J Am Chem Soc. 2002;124:14922–14933. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 32.Kalovidouris SA, Blixt O, Nelson A, Vidal S, Turnbull WB, Paulson JC, Stoddart JF. Chemically defined sialoside scaffolds for investigation of multivalent interactions with sialic acid binding proteins. J Org Chem. 2003;68:8485–8493. doi: 10.1021/jo030203g. [DOI] [PubMed] [Google Scholar]
- 33.Kitov PI, Shimizu H, Homans SW, Bundle DR. Optimization of tether length in nonglycosidically linked bivalent ligands that target sites 2 and 1 of a Shiga-like toxin. J Am Chem Soc. 2003;125:3284–3294. doi: 10.1021/ja0258529. [DOI] [PubMed] [Google Scholar]
- 34.Ramström O, Lohmann S, Bunyapapiboonsri T, Lehn JM. Dynamic combinatorial carbohydrate libraries: probing the binding site of the concanavalin A lectin. Chem Eur J. 2004;11:1711–1715. doi: 10.1002/chem.200305551. [DOI] [PubMed] [Google Scholar]
- 35.Thoma G, Katopodis AG, Voelcker N, Duthaler RO, Streiff MB. Novel glycodendrimers self-assemble to nanoparticles which function as polyvalent ligands in vitro and in vivo. Angew Chem, Int Ed. 2002;41:3195–3198. doi: 10.1002/1521-3773(20020902)41:17<3195::AID-ANIE3195>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 36.Jule E, Nagasaki Y, Kataoka K. Lactose-installed poly(ethylene glycol)-poly(D,L-lactide) block copolymer miscelles exhibit fast-rate binding and high affinity toward a protein bed stimulating a cell surface. A surface plasmon resonance study. Bioconjugate Chem. 2003;14:177–186. doi: 10.1021/bc025598+. [DOI] [PubMed] [Google Scholar]
- 37.Harada A. Cyclodextrin-based molecular machines. Acc Chem Res. 2001;34:456–464. doi: 10.1021/ar000174l. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann W, Keller B, Wenz G. Kinetics and thermodynamics of inclusion of ionene-6,10 in α-cyclodextrin in an aqueous solution. Macromolecules. 1997;30:4966–4972. [Google Scholar]
- 39.Wenz G, Han BH, Müller A. Cyclodextrin rotaxanes and polyrotaxanes. Chem Rev. 2006;106:782–817. doi: 10.1021/cr970027+. [DOI] [PubMed] [Google Scholar]
- 40.Bourne Y, Bolgiano B, Liao DI, Strecker G, Cantau P, Herzberg O, Feizi T, Cambillau C. Crosslinking of mammalian lectin (galectin-1) by complex biantennary saccharides. Nature Struct Biol. 1994;1:863–870. doi: 10.1038/nsb1294-863. [DOI] [PubMed] [Google Scholar]
- 41.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 42.He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem. 2004;279:4705–4712. doi: 10.1074/jbc.M311183200. [DOI] [PubMed] [Google Scholar]
- 43.Rubinstein N, Alverez M, Zwirnerm NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection: a potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 44.Pieters RJ. Inhibition and detection of galectins. ChemBioChem. 2006;7:721–728. doi: 10.1002/cbic.200600011. [DOI] [PubMed] [Google Scholar]
- 45.Lui FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 46.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez JD, Baum LG. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology. 2002;12:127R–136R. doi: 10.1093/glycob/cwf081. [DOI] [PubMed] [Google Scholar]
- 48.Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572:285–293. doi: 10.1016/s0304-4165(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 49.Stillmann BN, Mischel PS, Baum LG. New roles for galectins in brain tumors – from prognostic markers to therapeutic targets. Brain Pathol. 2005;15:124–132. doi: 10.1111/j.1750-3639.2005.tb00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada A, Adachi H, Kawaguchi Y, Okada M, Kamachi M. Complex formation of cyclodextrins with cationic polymers. Polym J (Tokyo) 1996;28:159–163. [Google Scholar]
- 51.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–256. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 52.Dam TK, Brewer CF. Carbohydrate–lectin cross-linking interactions: structural, thermodynamic, and biological studies. Methods Enzymol. 2003;362:455–486. doi: 10.1016/S0076-6879(03)01031-0. [DOI] [PubMed] [Google Scholar]
- 53.Pace KE, Hahn HP, Baum LG. Preparation of recombinant human galectin-1 and use in T-cell death assays. Methods Enzymol. 2003;363:499–518. doi: 10.1016/S0076-6879(03)01075-9. [DOI] [PubMed] [Google Scholar]
- 54.André S, Cejas Ortega PJ, Alamino Perez M, Roy R, Gabius HJ. Lactose-containing starburst denrimers: influence of dendrimer generation and binding-site orientation of receptors (plant/animal lectins and immunoglobulins) on binding properties. Glycobiology. 1999;9:1253–1261. doi: 10.1093/glycob/9.11.1253. [DOI] [PubMed] [Google Scholar]
- 55.André S, Pieters RJ, Vrasidas I, Kaltner H, Nishimura S-I, Liu FU, Liskamp RMJ, Gabius HJ. Wedgelike glycodendrimers as inhibitors of mammalian galectins to glycoproteins, lactose maxiclusters, and cell surface glyconjugates. ChemBioChem. 2001;2:822–830. doi: 10.1002/1439-7633(20011105)2:11<822::AID-CBIC822>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 56.Dimick SM, Powell SC, McMahon SA, Moothoo DN, Naismith JH, Toone EJ. On the meaning of affinity: cluster glycoside effects and concanavalin A. J Am Chem Soc. 1999;121:10286–1296. [Google Scholar]
- 57.Kramer RH, Karpen JW. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature. 1998;395:710–713. doi: 10.1038/27227. [DOI] [PubMed] [Google Scholar]
- 58.Kitov PI, Bundle DR. On the nature of the multivalency effect: a thermodynamic model. J Am Chem Soc. 2003;125:16271–16284. doi: 10.1021/ja038223n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed synthetic procedures and characterization of polyviologens PV-8 and PV-21 and an example of a light scattering experiment (Figure S1) are available at…