Abstract
Our work aimed to provide a topographical analysis of all known ionotropic P2X1–7 and metabotropic P2Y1,2,4,6,11–14 receptors that are present in vivo at the protein level in the basal ganglia nuclei and particularly in rat brain slices from striatum and substantia nigra. By immunohistochemistry-confocal and Western blotting techniques, we show that, with the exception of P2Y11,13 receptors, all other subtypes are specifically expressed in these areas in different amounts, with ratings of low (P2X5,6 and P2Y1,6,14 in striatum), medium (P2X3 in striatum and substantia nigra, P2X6,7 and P2Y1 in substantia nigra) and high. Moreover, we describe that P2 receptors are localized on neurons (colocalizing with neurofilament light, medium and heavy chains) with features that are either dopaminergic (colocalizing with tyrosine hydroxylase) or GABAergic (colocalizing with parvalbumin and calbindin), and they are also present on astrocytes (P2Y2,4, colocalizing with glial fibrillary acidic protein). In addition, we aimed to investigate the expression of P2 receptors after dopamine denervation, obtained by using unilateral injection of 6-hydroxydopamine as an animal model of Parkinson’s disease. This generates a rearrangement of P2 proteins: most P2X and P2Y receptors are decreased on GABAergic and dopaminergic neurons, in the lesioned striatum and substantia nigra, respectively, as a consequence of dopaminergic denervation and/or neuronal degeneration. Conversely, P2X1,3,4,6 on GABAergic neurons and P2Y4 on astrocytes augment their expression exclusively in the lesioned substantia nigra reticulata, probably as a compensatory reaction to dopamine shortage. These results disclose the presence of P2 receptors in the normal and lesioned nigro-striatal circuit, and suggest their potential participation in the mechanisms of Parkinson’s disease.
Keywords: Parkinson’s disease, Purinergic receptors, Rat brain, Tyrosine hydroxylase, γ-Aminobutyric acid, 6-Hydroxydopamine
Introduction
It is now well established that the arrangement of ionotropic P2X and metabotropic P2Y receptors [1, 2] on a cell membrane is a very dynamic process, often related to developmental or physiopathological conditions. Moreover, it is common knowledge that multiple P2 proteins are simultaneously recruited on a cell membrane for triggering biological functions. As a consequence, P2 receptors are rightly considered more than the sum of their single entities and must be therefore regarded as a complex network of cooperating receptors. Under this perspective, a numerical model was also introduced, the combinatorial receptor web model, which explains the biological efficacy of combining an assorted array of different P2 proteins on a given cell, in order to integrate, upgrade, guarantee and optimize specific receptor-dependent functions [3].
This trend of course applies to the central nervous system (CNS) as well, where in situ hybridization of P2 mRNA subtypes and immunohistochemistry of P2 proteins shows, for instance, wide but heterogeneous simultaneous distribution of both P2X [4–11] and P2Y [12–15] classes of receptors. In particular, P2X2,4,6 and P2Y1 subtypes are abundant and widespread approximately in the entire brain, while P2X1 protein is enriched in the cerebellum, P2X3 in the brain stem, and P2X7 is largely prejunctional. The hippocampus concurrently expresses all P2X and, moreover, P2Y1,2,4,6,12 receptor subtypes. Particularly in the basal ganglia (BG), neostriatal medium-spiny neurons and cholinergic interneurons highly express P2X2 and P2Y1 receptors, but it appears that they become functional only under certain, as yet unknown, conditions [16]. Moreover, P2X2 receptor protein was described in substantia nigra pars compacta (SNC) [17], whereas both protein and mRNA were described in SNC and striatum [18]. Finally, only very low levels of P2X4,6 mRNAs were detected in substantia nigra (SN) and striatum [19].
By functional analysis, ATP release was demonstrated from cultured embryonic neostriatal neurons [20], and ATP-evoked potassium currents in rat striatal neurons were shown to be mediated by P2 receptors [21]. ATP was also proved to increase extracellular dopamine levels in rat striatum through stimulation of P2Y subtypes [22], although it was claimed to inhibit dopamine release in the neostriatum [23]. Extracellular ATP via P2 receptors was finally reported to induce neurotoxicity in vitro [24] and in vivo [25] in the striatum. Besides P2 receptors on neurons, in BG there is also evidence of P2 receptors on, and release of ATP from, glial cells. P2Y12 subtype is present, for instance, on oligodendrocytes in striatum and SN [26], and P2X7 receptor is upregulated on microglia in striatum after middle cerebral artery occlusion [27]. In spite of these results, there is a general paucity of studies addressing the cellular distribution of all P2 receptor proteins in BG.
Our work thus aimed to provide the complete topographical analysis of known P2X and P2Y subtypes that are present in rat striatum and SN in vivo, and to investigate the dynamic presence of P2 proteins after the induction of experimental parkinsonism by dopamine-denervation achieved by using the unilateral 6-hydroxydopamine (6-OHDA) rat model. By upgrading the current map of P2 receptors expressed in the brain, our study discloses the potential impact of these receptors in the normal and lesioned nigro-striatal circuit.
Materials and methods
Histological procedures
Wistar rats (Harlan, Udine, Italy) were anesthetized by i.p. injections of sodium pentobarbital (60 mg/kg), and transcardially perfused with saline (0.9 % NaCl) followed by 4% paraformaldehyde, in phosphate buffer (PB, 0.1 M pH 7.4). Each brain was immediately removed, post-fixed in the same fixative for 2 h, and then transferred to 30% sucrose in PB at 4°C, until it sank. The experimental protocol used in this study was approved by the Italian Ministry of Health and was in agreement with the guidelines of the European Communities Council Directive of November 24, 1986 (86/609/EEC) for the care and use of laboratory animals. All efforts were made to minimize the number of animals used and their suffering.
Double immunofluorescence
Transverse sections (40-μm thick) were cut on a freezing microtome and were processed for double immunofluorescence studies. Non-specific binding sites were blocked with 10% normal donkey serum in 0.3% Triton X-100, in phosphate buffered saline (PBS) for 30 min at room temperature. The sections were incubated in a mixture of primary antisera for 24–48 h in 0.3% Triton X-100 in PBS. Rabbit anti-P2r (1:300, Alomone, Jerusalem, Israel) was used in combination with either mouse anti-calbindin-D-28K (1:200, Sigma, Mi, Italy), mouse anti-tyrosine hydroxylase (TH, 1:500, Sigma), mouse anti-parvalbumin (1:200, Chemicon International, Temecula, CA, USA), mouse anti-glial fibrillary acidic protein (GFAP) (1:400, Sigma), mouse anti-myelin basic protein (MBP, 1:200, Chemicon International), mouse anti-neurofilament H non-phosphorylated (SMI 32, 1:500, Sternberger Monoclonals, Lutherville, MD, USA), mouse anti-neurofilament H and M non-phosphorylated (SMI 33, 1:500, Sternberger Monoclonals), mouse anti-neurofilament 160 (NF160, 1:500, Sigma) or goat anti-neurofilament-L protein (NF-L, 1:100, Santa Cruz, Mi, Italy). The secondary antibodies used for double labeling were Cy3-conjugated donkey anti-rabbit IgG (1:100, red immunofluorescence, Jackson Immunoresearch, West Baltimore Pike, PA, USA), Cy2-conjugated donkey anti-mouse IgG (1:100, green immunofluorescence, Jackson Immunoresearch) or Cy2-conjugated donkey anti-goat IgG (1:100, green immunofluorescence, Jackson Immunoresearch).
The sections were washed in PBS three times for 5 min each, and then incubated for 3 h in a solution containing a mixture of the secondary antibodies in 1% normal donkey serum in PBS. After rinsing, the sections were mounted on slide glasses, allowed to air dry and coverslipped with gel/mount anti-fading medium (Biomeda, Foster City, CA, USA).
Confocal microscopy
Double- or triple-label immunofluorescence was analyzed by means of a confocal laser scanning microscope (CLSM) (LSM 510, Zeiss, Arese, Mi, Italy) equipped with argon laser emitting at 488 nm, helium/neon laser emitting at 543 nm, and helium/neon laser emitting at 633 nm. Specificity of the antibodies was positively proved by performing confocal analysis in the absence of the primary antibodies, but in the presence of either anti-rabbit or anti-mouse secondary antibodies. Specificity was further confirmed for the P2r antiserum by performing immunoreactions in the simultaneous presence of the P2r neutralizing immunogenic peptides.
Isolation of cerebral areas and protein extraction
Wistar rats were anesthetized by i.p. injections of sodium pentobarbital (60 mg/kg) and, after decapitation, brains were removed. Each brain was transversally cut on a vibratome (300 μm). The specific cerebral areas were isolated with the aid of a dissection microscope and homogenized in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS containing protease inhibitors). After short sonication, the homogenates were incubated on ice for 1 h and centrifuged at 14,000 r.p.m. for 10 min at 4°C. Protein quantification was performed in the supernatants by Bradford colorimetric assay (Biorad, Milan, Italy).
Western blot analysis
Equal amounts of cell lysate (20–30 μg of protein from each cerebral area) were separated by electrophoresis on 10–12% SDS-PAGE and transferred to nitrocellulose membranes Hybond-C extra (Amersham Biosciences, Cologno Monzese, Italy). The filters were pre-wetted in 5% non-fat milk in TBS-T (10 mM Tris pH 8, 150 mM NaCl, 0.1% Tween 20) and hybridized overnight with P2X1,2,4 antisera (Alomone, 1:500), P2X5 and P2Y4/14 (1:200), P2Y6 (1:300) or P2Y2 (1:400). The antisera were immunodetected with an anti-rabbit HRP-conjugated antibody (1:5,000) and developed by ECL chemiluminescence (Amersham Biosciences), using Kodak Image Station (KDS IS440CF).
Anti-P2r specificity
The polyclonal P2r antisera used in this study were raised against P2r highly purified peptides (identity confirmed by mass spectrography and amino acid analysis, as indicated in the certificate of analysis provided by the manufacturer), corresponding to specific epitopes not present in any other known protein. The specificity of the P2r signals was moreover assessed by incubating Western blots either in the absence of the primary antiserum, or in the presence of the primary antiserum together with the neutralizing P2r immunogenic peptides (μg protein ratio 1:1 between peptide and antiserum).
6-OHDA lesion and Nissl staining
Deeply anesthetized rats (45 days old, about 150 g body weight) were injected with 8 μg/4 μl 6-OHDA in saline 0.1% ascorbic acid in the medial forebrain bundle (stereotaxic coordinates ap = −4.4; l = +1.2; vd = −7.8, see also Paxinos et al. [28]) at a rate of 0.38 μl/min. Fifteen days later, the lesioned rats were tested with 0.05 mg/kg s.c. of the D1/D2 dopamine agonist apomorphine, in order to verify the efficacy of the 6-OHDA lesion, and contralateral turns to the lesion were counted for 40 min. Only those rats that made at least 200 contralateral turns were used for the study. It has been previously demonstrated that rats meeting this screening criterion have greater than 95% depletion of striatal dopamine [29]. At 1.5 months after the 6-OHDA lesion, rats were used for immunohistological experiments (n = 3). In order to evaluate cell damage, 40-μm rat brain sections were mounted onto gelatinized slides. They were dehydrated through alcohols, and then rehydrated and stained in 2% cresyl violet for 45 min. Following deionized water rinses, the slides were dehydrated in a standard alcohol series, cleared in xylene, and coverslipped.
Results
P2X and P2Y receptor proteins in rat striatum
We describe in this work the cellular and subcellular in vivo distribution of P2X and P2Y receptors in transverse sections of adult rat striatum, showing by double immunofluorescence confocal analysis that the various P2 subtype proteins are distinguished by different degrees of expression and are not uniformly distributed throughout the entire tissue (Fig. 1).
Fig. 1.
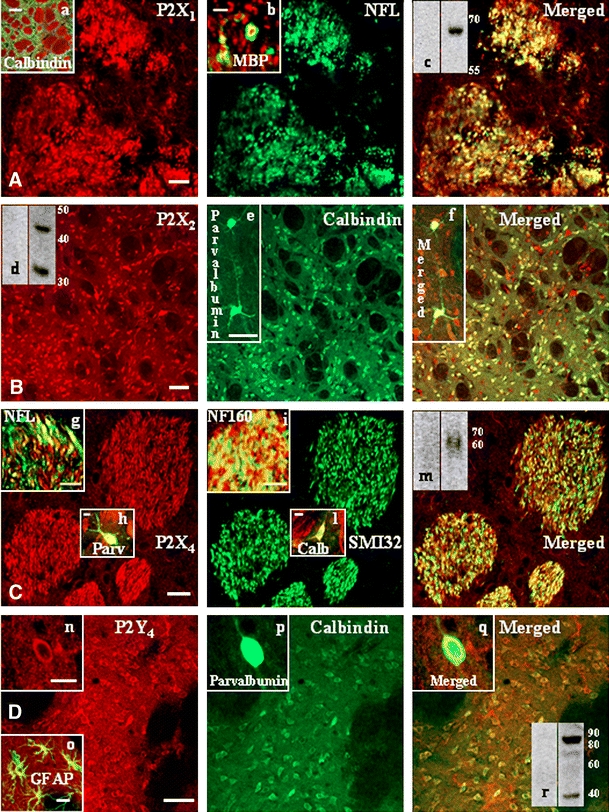
P2X and P2Y receptor proteins in rat striatum. Transverse sections through the striatum of adult rats were processed for double immunofluorescence studies. Rabbit polyclonal antisera against P2X1,2,4 and P2Y4 receptors (red Cy3 immunofluorescence) were used in combination with antibodies against neuronal or glial markers (green Cy2 immunofluorescence). Panel A P2X1: confocal images illustrate clear colocalization of P2X1 receptor with neurofilament-light protein (NF-L). The merged field of inset a shows absence of colocalization between the neuronal GABAergic marker calbindin (green) (a calcium-binding protein expressed mainly in medium spiny neurons of the striatum) and P2X1 receptor (red). The merged field of inset b shows the merged field of P2X1 (red) and MBP (green) overlapping immunoreactivities at higher magnification. Panel B P2X2: double immunofluorescence demonstrates that P2X2 receptor immunoreactivity (red) colocalizes with calbindin protein (green). The insets e and f show colocalization with the neuronal GABAergic marker parvalbumin (green) (a calcium-binding protein that is expressed in interneurons of the striatum). Panel C P2X4: red immunofluorescence for P2X4 protein merges with the green signals of the three types of neurofilament proteins: NF-L (inset g, merged field), NF160 (inset i, merged field) and SMI 32 and, moreover, with parvalbumin (inset h, merged field) and calbindin (inset i, merged field). Panel D P2Y4: red P2Y4 immunoreactivity is present on calbindin-positive neurons (green), on parvalbumin-positive neurons (green) (insets n–q), and on GFAP-positive astrocytes (inset o, merged field). Western blot analysis also confirms the presence of receptor proteins P2X1,2,4 (insets c in panel A, d in panel B, m in panel C, respectively) and P2Y4 (inset r in panel D) in striatum. Specificity of the P2 receptor signals was assessed by incubations of the primary antisera with the corresponding neutralizing immunogenic peptides (μg protein ratio 1:1 between peptide and antiserum). Scale bars are 10 μm in A; 100 μm in inset a; 2 μm in inset b; 50 μm in B and in insets e and f; 20 μm in C; 10 μm in insets h, i, l; 5 μm in inset g; 50 μm in D; 5 μm in inset n; and 20 μm in inset o. Similar results were obtained in at least four independent experiments
In particular, a strong P2X1 receptor immunoreactivity (red) confers a patchy appearance to the striatum, being localized mainly in white matter, while sparing the projecting calbindin-positive GABAergic neurons that are highly enriched in gray matter (Fig. 1A, inset a, green). Moreover, P2X1 protein immunofluorescence is present on NF-L positive, transversally oriented neuronal fibers, although the merged field provides only partial colocalization between the two signals (Fig. 1A). In addition, the high magnification analysis (Fig. 1A, inset b) of P2X1 (red) and MBP (green) immunoreactive signals shows that P2X1 receptor is surrounded by MBP, proving the presence of P2X1 protein on myelinated fibers. Due to the close vicinity of the two signals, overlapping yellow immunofluorescence is also observed. Finally, P2X1 receptor in striatum is recognized by Western blot analysis as a single protein band of 60–65 kDa, additionally abolished in the presence of the P2X1 receptor–neutralizing immunogenic peptide (Fig. 1A, inset c).
Conversely, an abundant P2X2 receptor immunoreactivity (red) is found in gray matter of striatum (Fig. 1B), while sparing the bundles of white matter. Specific receptor immunolabeling is present not only on the highly expressed calbindin-positive projecting GABAergic neurons, but also on the fewer parvalbumin-positive GABAergic interneurons (Fig. 1B, insets e, f). By Western blot analysis, we show that P2X2 receptor is present in striatum under two isoforms of about 45 and 32 kDa, furthermore, it is abolished in the presence of the P2X2 receptor–neutralizing immunogenic peptide (Fig. 1B, inset d).
P2X3 receptor immunostaining in striatum is of medium intensity (Table 1), and mainly localizes on GABAergic neurons of gray matter (data not shown).
Table 1.
Map of P2 receptor proteins in striatum and substantia nigra
| Striatum | Substantia nigra | |
|---|---|---|
| P2X1 | +++ | +++ |
| P2X2 | +++ | +++ |
| P2X3 | ++ | ++ |
| P2X4 | +++ | +++ |
| P2X5 | + | +++ |
| P2X6 | + | ++ |
| P2X7 | ++ | ++ |
| P2X1 | + | ++ |
| P2X2 | +++ | +++ |
| P2X4 | +++ | +++ |
| P2X6 | + | +++ |
| P2X11 | – | – |
| P2X12 | +++ | +++ |
| P2X13 | – | – |
| P2X14 | – | +++ |
Relative abundance of all P2X and P2Y receptor proteins was analyzed by confocal immunofluorescence microscopy, as described in “Materials and methods”. The intensity of the specific immunostaining was scored as follow: – = not detected; + = just sufficient to evaluate presence and outline of positive cells; ++ = adequate to assess morphological features of cell bodies and/or cellular processes; +++ = very bright
P2X4 receptor signal is instead very copious in white matter, although present on a few fibers of gray matter as well (Fig. 1C, red). It partially colocalizes with all types of heavy-, light- and medium-chain neurofilament proteins (merged fields): SMI 32 (green), NF-L (inset g), and NF160 (inset i). Moreover, we find P2X4 protein also on GABAergic interneurons (inset h) and GABAergic spiny neurons (inset l). By Western blot analysis, we demonstrate that P2X4 receptor is present in striatum as a single band of about 60 kDa, moreover, it is abolished in the presence of the P2X4 receptor–neutralizing immunogenic peptide (Fig. 1C, inset m).
P2X5,6,7 and P2Y1 receptor immunoreactivities in striatum are very weak (Table 1) in gray matter, although totally absent from white matter under our experimental conditions (data not shown).
The P2Y2 receptor is highly expressed in striatum on axons of white matter and astrocytes of gray matter (Table 1). Moreover, it is detected as a double protein band in the 55–65 kDa range (data not shown).
A strong P2Y4 receptor immunoreactivity is present only in gray matter of striatum, localized on both types of GABAergic neurons: calbindin-positive (Fig. 1D) and parvalbumin-positive (insets n–q). Nevertheless, the receptors are also widespread throughout the striatum on astrocytes, as shown by colocalization with the GFAP marker (inset o). By Western blot analysis, we prove that P2Y4 receptor is present in striatum as a double band of about 42 and 85 kDa (inset r), likely corresponding to the monomeric and dimeric aggregation states of the receptor [30, 31].
While P2Y6 receptor is barely detectable (Table 1) on GABAergic neurons in striatum (data not shown), P2Y11,13,14 receptor proteins were not identified by any means under our experimental conditions (Table 1). Finally, P2Y12 receptor in striatum (Table 1) is abundantly expressed only on oligodendrocytes and myelin sheets, as previously shown [26].
P2X and P2Y receptor proteins in substantia nigra
We conducted a parallel analysis on the cellular and subcellular in vivo distribution of P2X and P2Y receptors in transverse sections of adult rat SN. We showed by double immunofluorescence confocal analysis that the different P2 receptor proteins possess more comparable levels of expression with respect to the striatum, and are also more uniformly, although differently, distributed throughout the entire SNC and SNR (Fig. 2). In particular, strong signals for ionotropic P2X2,5 (red, Fig. 2A,B), P2X1,4 (Table 1), metabotropic P2Y6,14 (red, Fig. 2C,D) and P2Y4 (Table 1), or moderate signals for P2X3,6 and P2Y1 receptors (Table 1) are present on dopaminergic neurons (TH-positive) of SNC. Moreover, P2Y2 and P2Y12 receptors are abundantly expressed in SN (Table 1), but P2Y2 is expressed on axons and astrocytes, and P2Y12 only on oligodendrocytes and myelin sheets [26]. Conversely, in SNR, a weak P2X/Y receptor immunoreactivity is limited to sparse neuronal bodies, likely identified as GABAergic neurons by colocalization with parvalbumin (data not shown). The presence at the tissue level in SN of ionotropic P2X2,5 (insets a in panel A, and b in panel B of Fig. 2, respectively) and metabotropic P2Y6,14 (insets c in panel C, and d in panel D of Fig. 2, respectively) proteins is confirmed by Western blot analysis performed in all cases in the presence of specific receptor–neutralizing immunogenic peptides. Similarly to the striatum, immunoreactive signals for P2Y11,13 receptors were not identified by any means under our experimental conditions (Table 1).
Fig. 2.
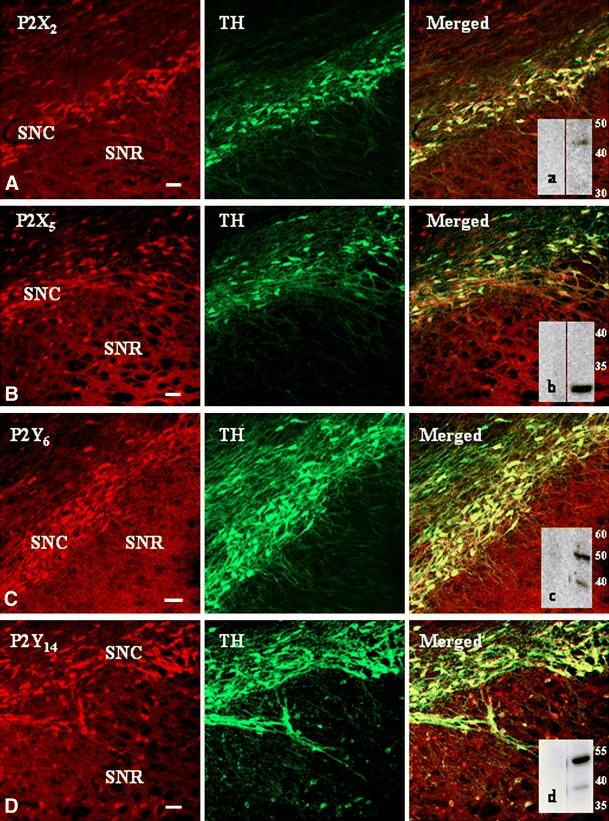
P2X and P2Y receptor proteins in rat substantia nigra. Double immunofluorescence visualized by confocal analysis was performed in transverse sections through the substantia nigra of adult rats. Strong signals for ionotropic P2X2,5 and metabotropic P2Y6,14 (red Cy3 immunofluorescence) are present on dopaminergic neurons (TH-positive, green Cy2 immunofluorescence) of substantia nigra pars compacta (SNC), whereas in substantia nigra pars reticolata (SNR) P2X/Y immunoreactivity is limited to sparse neuronal bodies. Western blot analysis confirms the presence in substantia nigra of receptor proteins P2X2,5 (insets a in panel A, and b in panel B, respectively) and P2Y6,14 (insets c in panel C, and d in panel D, respectively). Specificity of the P2 receptor signals was assessed by incubation of the primary antisera with the corresponding neutralizing immunogenic peptides (μg protein ratio 1:1 between peptide and antiserum).Scale bars in all panels are 50 μm. Similar results were obtained in at least four independent experiments
6-Hydroxydopamine modulates the expression of selected P2 receptors in striatum and substantia nigra
No contralateral rotation as a sign of motor deficit was reported in rats before being 6-OHDA-lesioned, but was instead detected after the lesion rotation (data not shown), together with loss of dopaminergic TH-positive neurons only from the ipsilateral hemisphere of SNC (Fig. 3A and insets a, b).
Fig. 3.
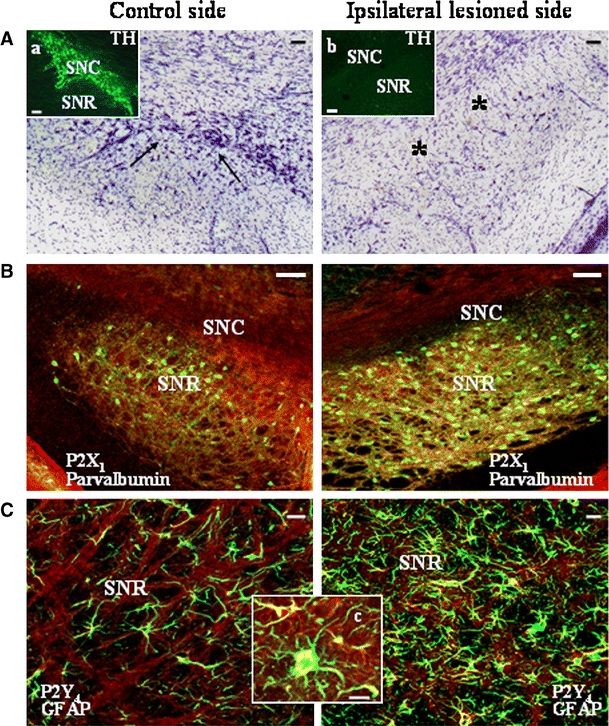
6-Hydroxydopamine modulates the expression of selected P2 receptor proteins in striatum and substantia nigra. Staining of rat substantia nigra after 6-hydroxydopamine treatment. Panel A Conventional microscopy images of Nissl staining shows several dopaminergic neurons (arrows) in the contralateral control hemisphere, which are lost (asterisks) in the ipsilateral lesioned hemisphere. Specific ipsilateral dopaminergic lesion of substantia nigra pars compacta (SNC) was also visualized by confocal TH-immunostaining (green) (insets a, b). Panel B Confocal merged yellow images show upregulation of P2X1 receptor protein (red) in parvalbumin-positive GABAergic neurons (green) in the lesioned side of substantia nigra pars reticolata (SNR) of 6-hydroxydopamine-treated rats. Panel C Confocal merged yellow images show a drastic increase in GFAP-positive astrocytes (green) in the lesioned side of 6-hydroxydopamine-treated rats and, correspondingly, an augment of P2Y4 signal (red) (inset c). Scale bars are 100 μm in A, B and in insets a, b; 20 μm in C; and 10 μm in inset c. Similar results were obtained in at least three independent experiments
Concomitantly, we prove that dopamine denervation in the 6-OHDA-lesioned rat generates a significant and selective rearrangement of P2 receptor proteins. Whereas the expression pattern and immunofluorescence intensities of P2X1,4, P2Y2 (colocalizing with all neurofilaments and present in white matter on fibers projecting from the cortex), and P2Y12 (present on oligodendrocytes of white matter) remain constant in both ipsi- and contralateral hemispheres after 6-OHDA treatment (as well as in control animals), all other P2X and P2Y receptors are decreased on parvalbumin- and calbindin-positive GABAergic neurons of deafferented ipsilateral striatum (but not contralateral and in control animals), as measured by semiquantitative analysis (Table 2) (n = 3).
Table 2.
Map of P2 receptor modulation after dopamine denervation
| Ipsilateral Striatum | Ipsilateral SN | |
|---|---|---|
| P2X1 | = | ↑GABA |
| P2X2 | ↓GABA | ↓TH |
| P2X3 | ↓GABA | ↓TH, ↑GABA |
| P2X4 | ↓GABA | ↓TH, ↑GABA |
| P2X5 | = | ↓TH |
| P2X6 | = | ↓TH, ↑GABA |
| P2X7 | = | = |
| P2X1 | = | ↓TH |
| P2X2 | = | = |
| P2X4 | ↓GABA | ↑GFAP |
| P2X6 | = | ↓TH |
| P2X11 | = | = |
| P2X12 | = | = |
| P2X13 | = | = |
| P2X14 | = | ↓TH |
Relative increase (↑) or decrease (↓) in P2X and P2Y receptor proteins analyzed by confocal immunofluorascence microscopy in striatum and SN after treatment in rat in vivo with 6-hydroxydopamine (ipsilateral), and in control (not lesioned) brain hemisphere (contralateral).TH= presence in dopaminergic neurons,GABA= presence in GABAergic neurons,GFAP= presence in astrocytes
Similarly, all P2X and P2Y receptors are lost in the lesioned (but not contralateral) substantia nigra pars compacta, consequent to the degeneration of the majority of TH-positive dopaminergic neurons (Table 2). Conversely, P2X1 (Fig. 3B) and P2X3,4,6 (Table 2) receptors present on GABAergic neurons, and P2Y4 receptors on astrocytes augment their expression only in ipsilateral substantia nigra pars reticulata adjacent to the lesioned pars compacta. In this same area, a phenomenon of astrogliosis is also induced, as detected by more abundant expression of GFAP-positive astrocytes (Fig. 3C).
Discussion
Because the roles of ATP in the CNS have received less attention until recently, often due to lack of appropriate research tools, our knowledge of the functional qualification of P2 receptors in the brain is limited, although rapidly improving. As a group of nuclei interconnected with cerebral cortex, thalamus and brainstem, and associated with a variety of functions, such as motor control, cognition, emotions and learning, the BG [32] is an area that deserves thorough analysis. Our work was aimed at mapping in vivo the presence of P2 receptor subtypes in the BG nuclei of striatum and SN by immunofluorescence-confocal and Western blotting techniques. The specificity of the highly sensitive molecular probes used for the detection of all known P2X and P2Y receptor proteins has been previously validated [33, 34]. In addition, we undertook an analysis that excluded possible cross-reactivity for all antisera used.
Our results not only establish that the majority of P2X (P2X1–7) and P2Y (P2Y1,2,4,6,11–14) receptors so far cloned from mammalian tissues are found in striatum and SN, but also prove their distinctive localization on neurons and/or glial cells. In detail we show that, with the exception of only P2Y11 and P2Y13 receptors (whose immunoreactivity was not identified by any means under our experimental conditions), all other subtypes are specifically localized in striatum and SN (both pars compacta and reticulata), although with different levels of expression, rated as low (P2X5,6 and P2Y1,6,14 in striatum), medium (P2X3 in striatum and SN; P2X6,7 and P2Y1 in SN) and high. Moreover, while we show a prevalence of P2 receptors on neurons (P2X1,4 and P2Y2 colocalizing with neurofilament light, medium and heavy chains) with features that are either dopaminergic (P2X2–5 and P2Y1,4,6,14 colocalizing with TH, in SN) or GABAergic (P2X2–4 and P2Y4 colocalizing with parvalbumin and calbindin, in striatum), we also describe their expression on astrocytes (P2Y2,4 in striatum and SN, colocalizing with GFAP), microglia (P2X7, colocalizing with OX42) [27], and oligodendrocytes (P2Y12, colocalizing with MBP and RIP) [26]. By confirming previous autoradiographic studies [35, 36], our results therefore prove the widespread but diversified P2-receptor protein distribution in striatum and SN, and extend to these nuclei the great level of biological complexity and molecular sophistication pertaining to P2 receptors [3].
Although the configuration of receptor subunits required for assembly into functional cation channels gated by extracellular ATP in different regions of the CNS comprising the BG is not known yet, colocalization of so many different P2X subtypes in striatum and SN is definitely compatible with heteromultimeric assembly of ionotropic subunits. Since a growing body of biochemical and biophysical evidence now indicates that the propensity to form homo- and especially hetero-multimers is frequent also for G protein-coupled receptors [37] comprising the P2Y subtypes [30, 31], the concurrent expression in striatum and SN of as many metabotropic receptors could explain once more a complex hetero-oligomeric architecture. Nevertheless, the biological phenomenon of redundancy could also justify the simultaneous presence of multiple P2 receptor subtypes in these nuclei, with the final outcome of increasing the structural and pharmacological heterogeneity of these brain regions. Finally, the composite architecture of P2 receptors that we depicted in striatum and SN might likely also signify a multipart mechanism of receptor cooperative behavior (Volonté et al., personal communication) that sustains the concomitant level of complexity of this brain area in several tasks, such as planning and modulation of movement pathways, cognitive processes involving executive functions, reward and addiction. These possibilities are, of course, not mutually exclusive.
Striatal neurons, including the most abundant medium spiny neurons, receive convergent synaptic modulation from nigral dopaminergic neurons and from cortical glutamatergic projections [38]. The present study showing that lesions of nigral dopaminergic neurons do not significantly affect purinergic receptors present on axons of striatum white matter, but do generate a significant overall decrease in P2X and P2Y receptor proteins from striatal spiny neurons and GABAergic interneurons, thus confirms and extends the involvement of P2 receptors and extracellular ATP to the cortex-basal ganglia circuit [21]. Since dopaminergic denervation affects not only the nigrostriatal dopaminergic pathway but, as a consequence, the corticostriatal glutamatergic pathway with an increase in glutamatergic transmission [39–41] and extracellular glutamate levels in the striatum [42], the reduced P2 receptor protein expression that we demonstrate in striatum gray matter could thus not only be a direct effect of the nigrostriatal inhibition, but also a cause of de-inhibitory mechanisms occurring in the corticostriatal circuit. In this regard, it is common knowledge that extracellular ATP participates in excitatory neurotransmission in the CNS [43], that release of extracellular ATP occurs in CNS under both normal and pathological conditions [44] and, not least, that glutamate release is induced by extracellular ATP in CNS glutamatergic neurons [45].
Neurons of the pars compacta responsible for dopamine production in the brain, which we have shown here to completely lose their array of P2 receptors as a consequence of neurodegeneration induced by 6-OHDA treatment, receive inhibiting signals also from neurons of the pars reticulata that produce GABA [46]. Loss of dopamine neurons in the SNC, one of the main pathological features of Parkinson’s disease leading to a marked reduction in dopamine function in the brain, thus also impedes the inhibitory pathway of SNR, with a consequent overactivation of GABAergic neurons. Our findings that specific expression of both ionotropic P2X1,3,4,6 receptors on GABAergic neurons and metabotropic P2Y4 receptors on astrocytes is remarkably increased in SNR after dopamine denervation thus probably reflects a parallel compensatory overreaction of GABAergic neurons to dopamine shortage. One possible explanation is that purinergic mechanisms might thus play a crucial role in the fine-tuned regulation not only of dopaminergic and glutamatergic cross-talk in striatum, as it occurs in nucleus accumbens [47], but also of GABAergic and dopaminergic interplay in SN, as it occurs in the mesolimbic neuronal circuit [48]. This is consistent with the overall versatile functions accomplished by P2 receptors in the CNS under both normal and pathological conditions [43, 44, 49] and, in particular, with the intermediary role in oligodendrocyte-to-neuron [26], Bergmann glia-to-neuron, and neuron-to-neuron communication [50] proposed for P2 receptors in various brain regions.
In summary, the importance of our work is twofold. We first provide the complete topographical analysis of all known P2X and P2Y receptor subtypes expressed in vivo at their protein levels in rat striatum and SN, which, when considered alongside functional studies, supports a key role for extracellular ATP as a cotransmitter/neuromodulator in these brain areas. Then, we prove that dopamine denervation in the 6-OHDA animal model of Parkinson’s disease generates a significant rearrangement of P2 receptor proteins in these nuclei, therefore disclosing the participation of P2 receptors in the lesioned nigro-striatal circuit. While requiring further investigation, our findings indicate a potential but noteworthy pharmacological and therapeutic novel outcome for Parkinson’s disease.
Acknowledgements
The research presented was supported by Cofinanziamento MIUR “Purinoceptors and Neuroprotection,” and a grant from Ministero della Salute RF05.105V.
References
- 1.Burnstock G (2007a) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797 [DOI] [PubMed]
- 2.Burnstock G (2007b) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483 [DOI] [PMC free article] [PubMed]
- 3.Volonté C, Amadio S, D’Ambrosi N, Colpi M, Burnstock G (2006) P2 receptor web: complexity and fine-tuning. Pharmacol Ther 112:264–280 [DOI] [PubMed]
- 4.Lê KT, Babinski K, Seguela P (1998) Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci 18:7152–7159 [DOI] [PMC free article] [PubMed]
- 5.Llewellyn-Smith IJ, Burnstock G (1998) Ultrastructural localization of P2X3 receptors in rat sensory neurons. NeuroReport 9:2245–2250 [DOI] [PubMed]
- 6.Loesch A, Burnstock G (1998) Electron-immunocytochemical localization of P2X1 receptors in the rat cerebellum. Cell Tissue Res 294:253–260 [DOI] [PubMed]
- 7.Xiang Z, Bo X, Burnstock G (1998) Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett 256:105–108 [DOI] [PubMed]
- 8.Nörenberg W, Illes P (2000) Neuronal P2X receptors: localisation and functional properties. Naunyn Schmiedebergs Arch Pharmacol 362:324–339 [DOI] [PubMed]
- 9.Rubio ME, Soto F (2001) Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci 21:641–653 [DOI] [PMC free article] [PubMed]
- 10.North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed]
- 11.Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304 [DOI] [PubMed]
- 12.Moran-Jimenez M, Matute C (2000) Immunohistochemical localization of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Brain Res 78:50–58 [DOI] [PubMed]
- 13.Moore D, Chambers J, Waldvogel H, Faull R, Emson P (2000) Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol 421:374–384 [DOI] [PubMed]
- 14.Laitinen JT, Uri A, Raidaru G, Miettinen R (2001) [35S]GTPgS autoradiography reveals a wide distribution of Gi/o-linked ADP receptors in the nervous system: close similarities with the platelet P2Y(ADP) receptor. J Neurochem 77:505–518 [DOI] [PubMed]
- 15.Burnstock G (2003) Purinergic receptors in the nervous system. In: Schwiebert EM (ed) Current topics in membranes. Purinergic receptors and signalling, vol. 54. Academic, San Diego, pp 307–368
- 16.Scheibler P, Pesic M, Franke H, Reinhardt R, Wirkner K, Illes P, Norenberg W (2004) P2X2 and P2Y1 immunofluorescence in rat neostriatal medium-spiny projection neurones and cholinergic interneurons is not linked to respective purinergic receptor function. Br J Pharmacol 143:119–131 [DOI] [PMC free article] [PubMed]
- 17.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R (1996) Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA 93:8063–8067 [DOI] [PMC free article] [PubMed]
- 18.Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF (1999) Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol 407:11–32 [DOI] [PubMed]
- 19.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G (1996) Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16:2495–2507 [DOI] [PMC free article] [PubMed]
- 20.Zhang J, Kornecki E, Jackman J, Ehrlich YH (1988) ATP secretion and extracellular protein phosphorylation by CNS neurons in primary culture. Brain Res Bull 21:459–464 [DOI] [PubMed]
- 21.Ikeuchi Y, Nishizaki T (1995) ATP-evoked potassium currents in rat striatal neurons are mediated by a P2 purinergic receptor. Neurosci Lett 190:89–92 [DOI] [PubMed]
- 22.Zhang YX, Yamashita H, Ohshita T, Sawamoto N, Nakamura S (1995) ATP increases extracellular dopamine level through stimulation of P2Y purinoceptors in the rat striatum. Brain Res 691:205–212 [DOI] [PubMed]
- 23.Trendelenburg AU, Bultmann R (2000) P2 receptor-mediated inhibition of dopamine release in rat neostriatum. Neuroscience 96:249–252 [DOI] [PubMed]
- 24.Amadio S, D’Ambrosi N, Cavaliere F, Murra B, Sancesario G, Bernardi G, Burnstock G, Volonté C (2002) P2 receptor modulation and cytotoxic function in cultured CNS neurones. Neuropharmacology 42:489–501 [DOI] [PubMed]
- 25.Ryu JK, Kim J, Choi SH, Oh YJ, Lee YB, Kim SU, Jin BK (2002) ATP-induced in vivo neurotoxicity in the rat striatum via P2 receptors. NeuroReport 13:1611–1615 [DOI] [PubMed]
- 26.Amadio S, Tramini G, Martorana A, Viscomi MT, Sancesario G, Bernardi G, Volontè C (2006) Oligodendrocytes express P2Y12 metabotropic receptor in adult rat brain. Neuroscience 141:1171–1180 [DOI] [PubMed]
- 27.Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volontè C, Bernardi G, Pedata F, Sancesario G (2006) P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 26:974–982 [DOI] [PubMed]
- 28.Paxinos G, Watson C, Pennisi M, Topple A (1985) Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods 13:139–143 [DOI] [PubMed]
- 29.Schwarting RK, Huston JP (1996) The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol 50:275–331 [DOI] [PubMed]
- 30.D’Ambrosi N, Iafrate M, Vacca F, Amadio S, Tozzi A, Mercuri NB, Volonté C (2006) The P2Y4 receptor forms homo-oligomeric complexes in several CNS and PNS neuronal cells. Purinergic Signal 2:575–582 [DOI] [PMC free article] [PubMed]
- 31.D’Ambrosi N, Iafrate M, Saba E, Rosa P, Volonté C (2007) Comparative analysis of P2Y(4) and P2Y(6) receptor architecture in native and transfected neuronal systems. Biochim Biophys Acta 1768:1592–1599 [DOI] [PubMed]
- 32.Fisone G, Håkansson K, Borgkvist A, Santini E (2007) Signaling in the basal ganglia: postsynaptic and presynaptic mechanisms. Physiol Behav. DOI10.1016/j.physbeh.2007.05.028 [DOI] [PubMed]
- 33.Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volontè C, Matteoli M, Abbracchio MP, Verderio C (2005) Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev 48:144–156 [DOI] [PubMed]
- 34.Fries JE, Goczalik IM, Wheeler-Schilling TH, Kohler K, Guenther E, Wolf S, Wiedemann P, Bringmann A, Reichenbach A, Francke M, Pannicke T (2005) Identification of P2Y receptor subtypes in human muller glial cells by physiology, single cell RT-PCR, and immunohistochemistry. Invest Ophthalmol Vis Sci 46:3000–3007 [DOI] [PubMed]
- 35.Bo X, Burnstock G (1994) Distribution of [3H]alpha,beta-methylene ATP binding sites in rat brain and spinal cord. NeuroReport 5:1601–1604 [DOI] [PubMed]
- 36.Balcar VJ, Li Y, Killinger S, Bennett MR (1995) Autoradiography of P2x ATP receptors in the rat brain. Br J Pharmacol 115:302–306 [DOI] [PMC free article] [PubMed]
- 37.Javitch JA (2004) The ants go marching two by two: oligomeric structure of G-protein-coupled receptors. Mol Pharmacol 66:1077–1082 [DOI] [PubMed]
- 38.Calabresi P, Picconi B, Tozzi A, Di Filippo M (2007) Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 30:211–219 [DOI] [PubMed]
- 39.Calabresi P, Mercuri NB, Sancesario G, Bernardi G (1993) Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain 116:433–452 [DOI] [PubMed]
- 40.Onn SP, West AR, Grace AA (2000) Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci 23(10 Suppl):S48–S56 [DOI] [PubMed]
- 41.Picconi B, Pisani A, Centonze D, Battaglia G, Storto M, Nicoletti F, Bernardi G, Calabresi P (2002) Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment. Brain 125(Pt 12):2635–2645 [DOI] [PubMed]
- 42.Robelet S, Melon C, Guillet B, Salin P, Kerkerian-Le Goff L (2004) Chronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson’s disease. Eur J Neurosci 20:1255–1266 [DOI] [PubMed]
- 43.Volonté C, Amadio S, Cavaliere F, D’Ambrosi N, Vacca F, Bernardi G (2003) Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord 2:403–412 [DOI] [PubMed]
- 44.Franke H, Illes P (2006) Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther 109:297–324 [DOI] [PubMed]
- 45.Merlo D, Volonté C (1996) Binding and functions of extracellular ATP in cultured cerebellar granule neurons. Biochem Biophys Res Comm 225:907–914 [DOI] [PubMed]
- 46.Tepper JM, Lee CR (2007) GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res 160:189–208 [DOI] [PubMed]
- 47.Krügel U, Kittner H, Illes P (2001) Mechanisms of adenosine 5′-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Synapse 39:222–232 [DOI] [PubMed]
- 48.Krügel U, Kittner H, Franke H, Illes P (2003) Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse 47:134–142 [DOI] [PubMed]
- 49.Koles L, Furst S, Illes P (2005) P2X and P2Y receptors as possible targets of therapeutic manipulations in CNS illnesses. Drug News Perspect 18:85–101 [DOI] [PubMed]
- 50.Amadio S, Vacca F, Martorana A, Sancesario G, Volonté C (2007) P2Y1 receptor switches to neurons from glia in juvenile versus neonatal rat cerebellar cortex. BMC Dev Biol 7(1):77 [DOI] [PMC free article] [PubMed]


