Abstract
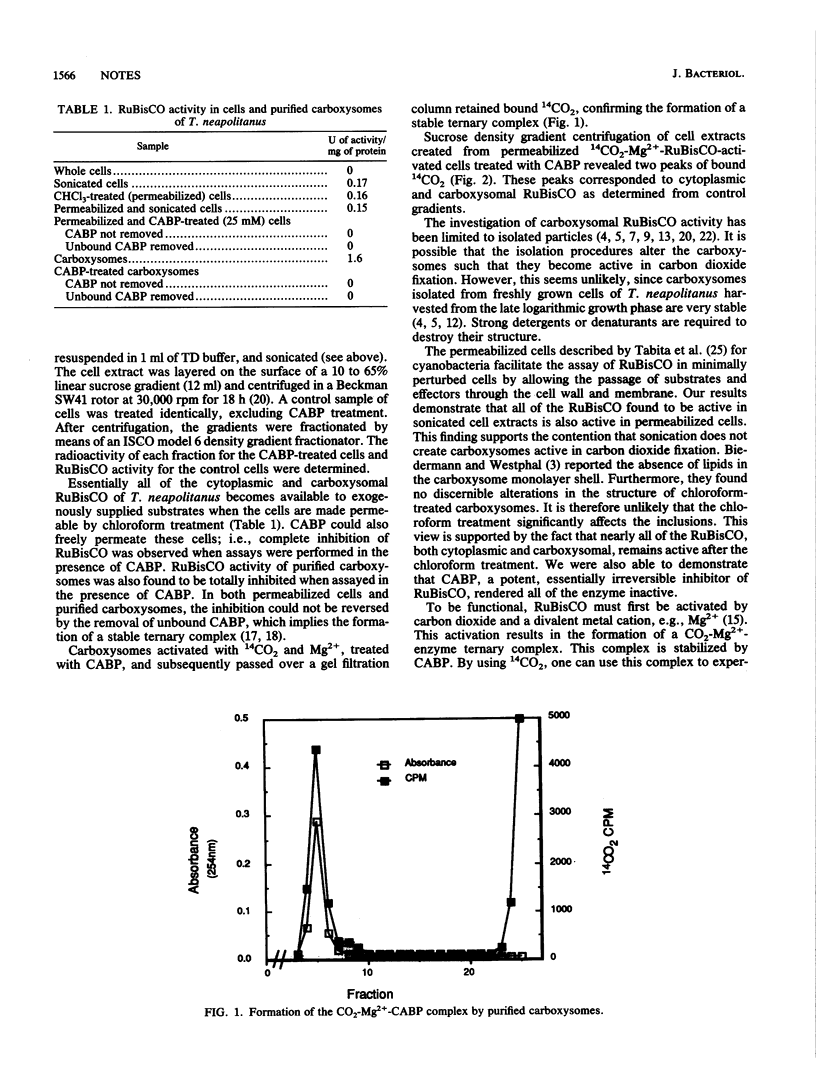
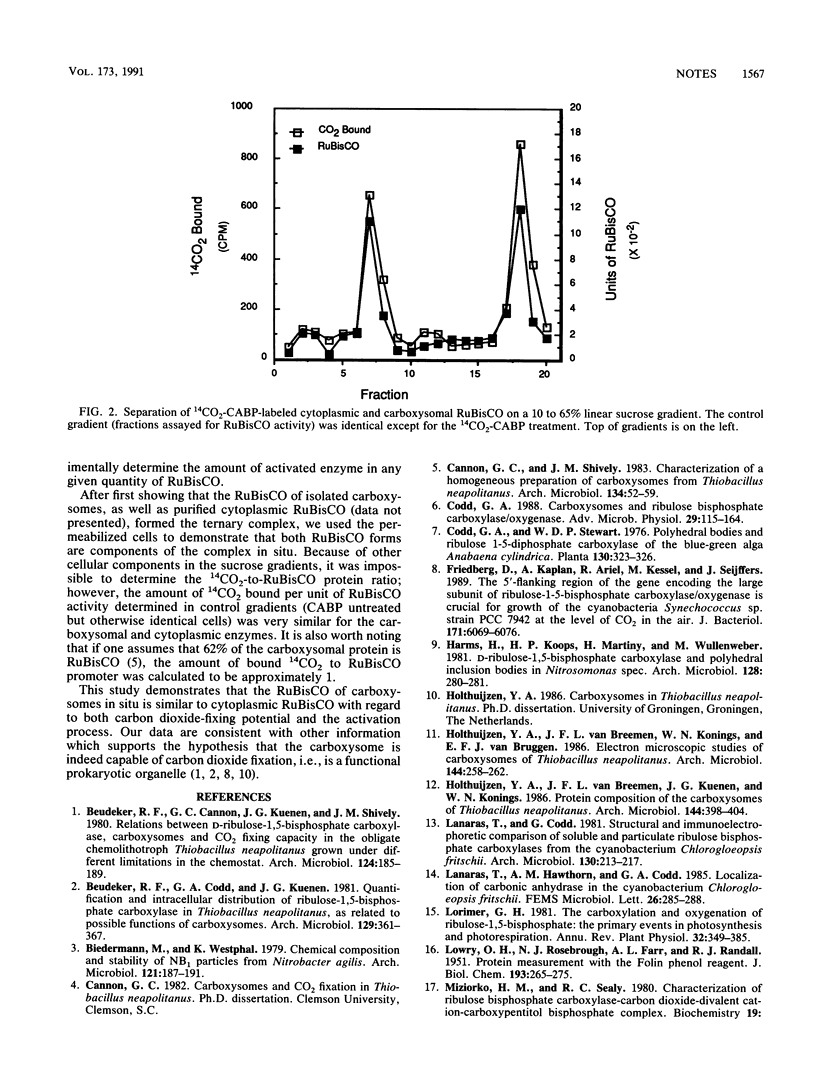
Cells permeabilized with chloroform yielded ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) activities nearly equal to those of cell extracts, thus indicating that both cytoplasmic and carboxysomal RuBisCO are functional in situ. The carboxysomal and cytoplasmic RuBisCO both form the CO2-Mg2(+)-enzyme ternary complex, as evidenced by stabilization with 2-C-carboxy-D-arabinitol-1,5-bisphosphate (CABP), a potent competitive inhibitor of RuBisCO. The data are consistent with the hypothesis that the carboxysome is functional in carbon dioxide fixation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Codd G. A. Carboxysomes and ribulose bisphosphate carboxylase/oxygenase. Adv Microb Physiol. 1988;29:115–164. doi: 10.1016/s0065-2911(08)60347-1. [DOI] [PubMed] [Google Scholar]
- Friedberg D., Kaplan A., Ariel R., Kessel M., Seijffers J. The 5'-flanking region of the gene encoding the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is crucial for growth of the cyanobacterium Synechococcus sp. strain PCC 7942 at the level of CO2 in air. J Bacteriol. 1989 Nov;171(11):6069–6076. doi: 10.1128/jb.171.11.6069-6076.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Shively J. M., Ball F. L., Kline B. W. Electron microscopy of the carboxysomes (polyhedral bodies) of Thiobacillus neapolitanus. J Bacteriol. 1973 Dec;116(3):1405–1411. doi: 10.1128/jb.116.3.1405-1411.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Ball F., Brown D. H., Saunders R. E. Functional organelles in prokaryotes: polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus. Science. 1973 Nov 9;182(4112):584–586. doi: 10.1126/science.182.4112.584. [DOI] [PubMed] [Google Scholar]
- Shively J. M., Bock E., Westphal K., Cannon G. C. Icosahedral inclusions (carboxysomes) of Nitrobacter agilis. J Bacteriol. 1977 Nov;132(2):673–675. doi: 10.1128/jb.132.2.673-675.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Bryant D. A., Fuller R. C., Konopka A. E., Stevens S. E., Jr, Strohl W. R. Functional inclusions in prokaryotic cells. Int Rev Cytol. 1988;113:35–100. doi: 10.1016/s0074-7696(08)60846-3. [DOI] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Caruso P., Whitman W. Facile assay of enzymes unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5-bisphosphate carboxylase. Anal Biochem. 1978 Feb;84(2):462–472. doi: 10.1016/0003-2697(78)90064-7. [DOI] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


