Abstract
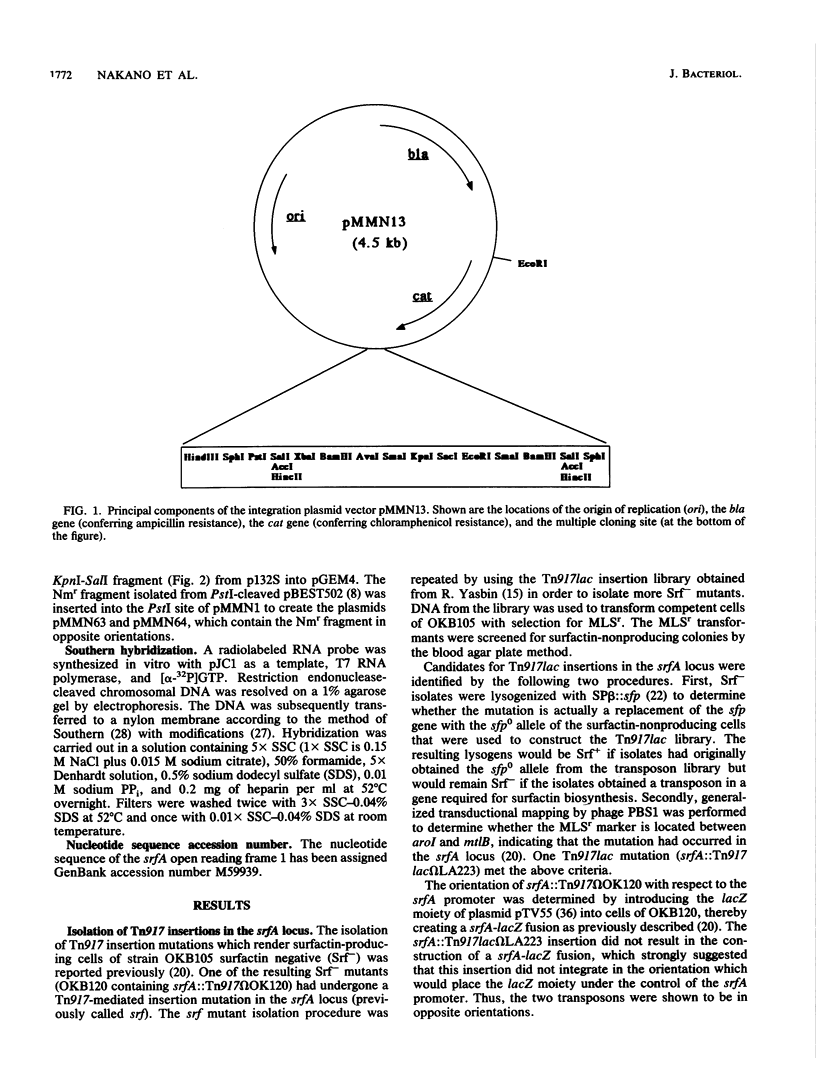
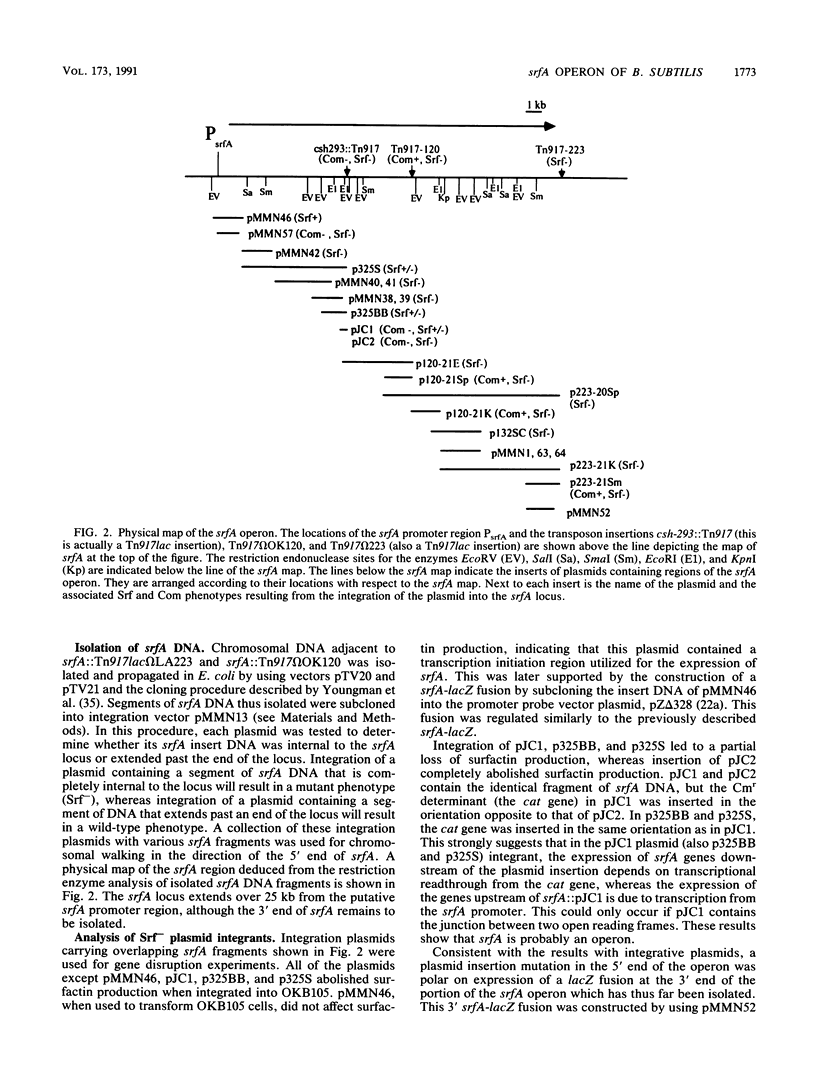
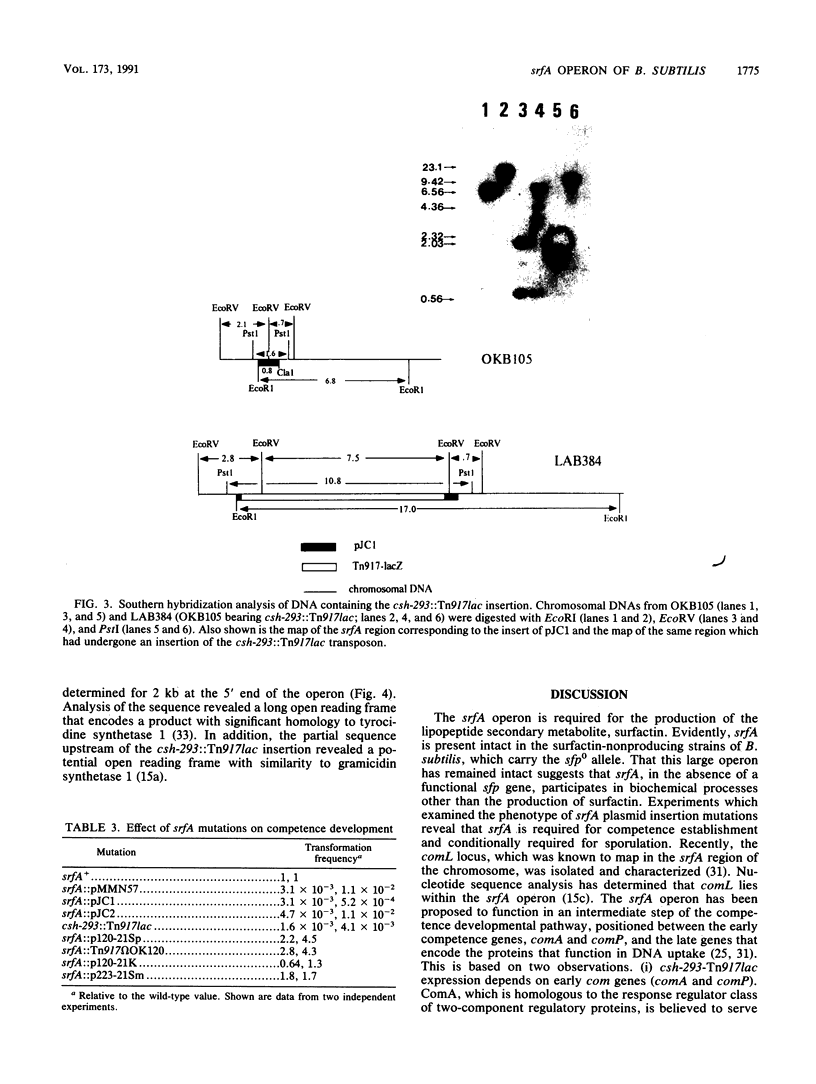
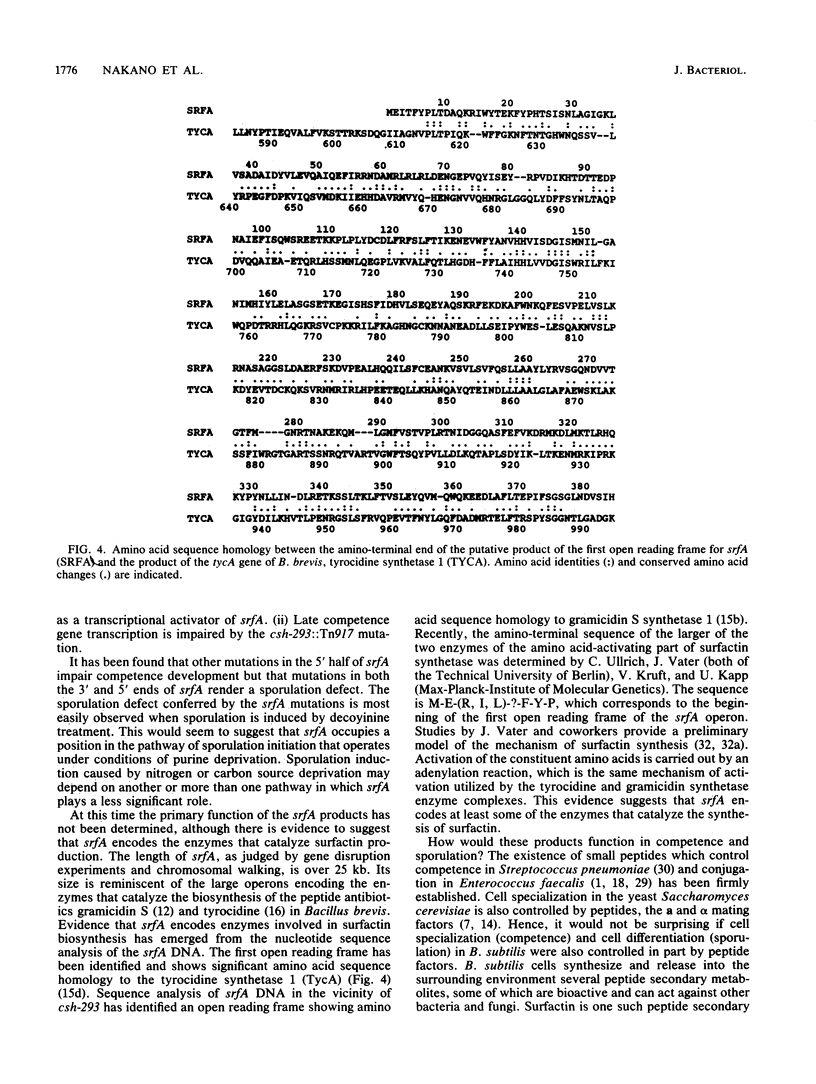
The srfA locus of Bacillus subtilis is defined by a transposon Tn917 insertion and is required for production of the peptide secondary metabolite surfactin. The srfA locus was isolated by cloning the DNA flanking srfA::Tn917 insertions followed by chromosome walking. The cloned region is an operon of over 25 kb which covers the transcription initiation region but not the intact 3' end of srfA. csh-293, which was previously identified as a Tn917lac mutation that impairs competence development and causes a conditional defect in sporulation, was known to be located in the vicinity of the srfA locus within the B. subtilis genome. The csh-293::Tn917lac mutation was discovered to cause a defect in surfactin production and was shown to be located in the srfA operon by its cotransformation with srfA mutations and by Southern hybridization analysis. Insertion mutations in srfA, created by the chromosomal integration of plasmids bearing overlapping srfA DNA fragments, were examined for their effects on surfactin production, competence, and sporulation. All three processes were found to require the intact 5' half of the srfA operon, whereas the 3' half of srfA was found to be required for sporulation and surfactin production but not competence. These experiments show that srfA gene products function in B. subtilis cell specialization and differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freese E. B., Vasantha N., Freese E. Induction of sporulation in developmental mutants of Bacillus subtilis. Mol Gen Genet. 1979 Feb 16;170(1):67–74. doi: 10.1007/BF00268581. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen N., Weinrauch Y., Dubnau D. A. Cloning and characterization of the regulatory Bacillus subtilis competence genes comA and comB. J Bacteriol. 1989 Oct;171(10):5354–5361. doi: 10.1128/jb.171.10.5354-5361.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D. J., Yang M., Ferrari E. Localization of Bacillus subtilis sacU(Hy) mutations to two linked genes with similarities to the conserved procaryotic family of two-component signalling systems. J Bacteriol. 1988 Nov;170(11):5102–5109. doi: 10.1128/jb.170.11.5102-5109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989 Dec 14;342(6251):749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- Itaya M., Kondo K., Tanaka T. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 1989 Jun 12;17(11):4410–4410. doi: 10.1093/nar/17.11.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks K. J., Healy J., Losick R., Grossman A. D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989 Aug;171(8):4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H., Gruber M., Venema G. Stimulation of the development of competence by culture fluids in Bacillus subtilis transformation. Biochim Biophys Acta. 1972 Mar 14;262(2):189–199. doi: 10.1016/0005-2787(72)90232-8. [DOI] [PubMed] [Google Scholar]
- Katz E., Demain A. L. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977 Jun;41(2):449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krätzschmar J., Krause M., Marahiel M. A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989 Oct;171(10):5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst F., Debarbouille M., Msadek T., Young M., Mauel C., Karamata D., Klier A., Rapoport G., Dedonder R. Deduced polypeptides encoded by the Bacillus subtilis sacU locus share homology with two-component sensor-regulator systems. J Bacteriol. 1988 Nov;170(11):5093–5101. doi: 10.1128/jb.170.11.5093-5101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Lyle M. J., Yasbin R. E. DNA-damage-inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6201–6205. doi: 10.1073/pnas.82.18.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittenhuber G., Weckermann R., Marahiel M. A. Gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol. 1989 Sep;171(9):4881–4887. doi: 10.1128/jb.171.9.4881-4887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modest B., Marahiel M. A., Pschorn W., Ristow H. Peptide antibiotics and sporulation: induction of sporulation in asporogenous and peptide-negative mutants of Bacillus brevis. J Gen Microbiol. 1984 Apr;130(4):747–755. doi: 10.1099/00221287-130-4-747. [DOI] [PubMed] [Google Scholar]
- Mori M., Tanaka H., Sakagami Y., Isogai A., Fujino M., Kitada C., White B. A., An F. Y., Clewell D. B., Suzuki A. Isolation and structure of the Streptococcus faecalis sex pheromone, cAM373. FEBS Lett. 1986 Sep 29;206(1):69–72. doi: 10.1016/0014-5793(86)81342-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee P. K., Paulus H. Biological function of gramicidin: studies on gramicidin-negative mutants. Proc Natl Acad Sci U S A. 1977 Feb;74(2):780–784. doi: 10.1073/pnas.74.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Marahiel M. A., Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol. 1989 Oct;171(10):5347–5353. doi: 10.1128/jb.171.10.5347-5353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. Molecular biology of antibiotic production in Bacillus. Crit Rev Biotechnol. 1990;10(3):223–240. doi: 10.3109/07388559009038209. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Ehrlich S. D. In vitro genetic labeling of Bacillus subtilis cryptic plasmid pHV400. Plasmid. 1979 Jan;2(1):48–58. doi: 10.1016/0147-619x(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Piret J. M., Demain A. L. Sporulation and spore properties of Bacillus brevis and its gramicidin S-negative mutant. J Gen Microbiol. 1983 May;129(5):1309–1316. doi: 10.1099/00221287-129-5-1309. [DOI] [PubMed] [Google Scholar]
- Roggiani M., Hahn J., Dubnau D. Suppression of early competence mutations in Bacillus subtilis by mec mutations. J Bacteriol. 1990 Jul;172(7):4056–4063. doi: 10.1128/jb.172.7.4056-4063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Mori M., Sakagami Y., Isogai A., Fujino M., Kitada C., Craig R. A., Clewell D. B. Isolation and structure of bacterial sex pheromone, cPD1. Science. 1984 Nov 16;226(4676):849–850. doi: 10.1126/science.6436978. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Mosser J. L. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A. 1966 Jan;55(1):58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckermann R., Fürbass R., Marahiel M. A. Complete nucleotide sequence of the tycA gene coding the tyrocidine synthetase 1 from Bacillus brevis. Nucleic Acids Res. 1988 Dec 23;16(24):11841–11841. doi: 10.1093/nar/16.24.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrauch Y., Guillen N., Dubnau D. A. Sequence and transcription mapping of Bacillus subtilis competence genes comB and comA, one of which is related to a family of bacterial regulatory determinants. J Bacteriol. 1989 Oct;171(10):5362–5375. doi: 10.1128/jb.171.10.5362-5375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P., Perkins J. B., Losick R. A novel method for the rapid cloning in Escherichia coli of Bacillus subtilis chromosomal DNA adjacent to Tn917 insertions. Mol Gen Genet. 1984;195(3):424–433. doi: 10.1007/BF00341443. [DOI] [PubMed] [Google Scholar]