Abstract
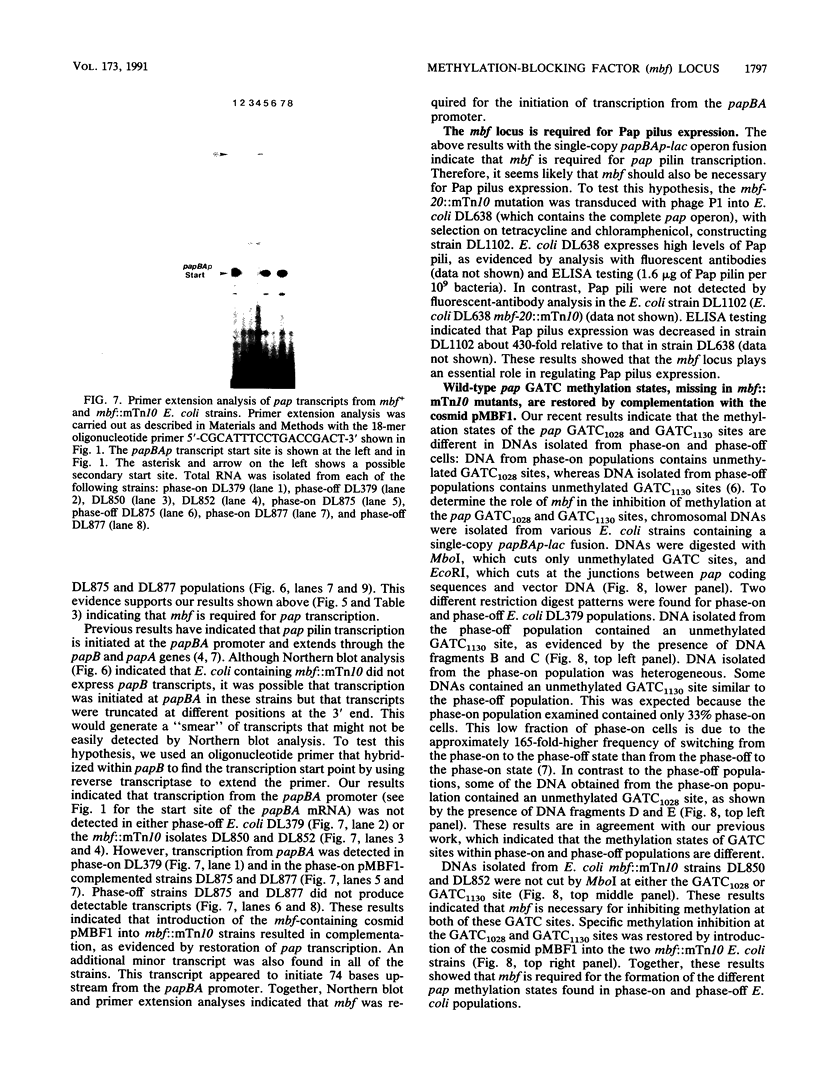
Transcription of the pyelonephritis-associated pilus (pap) operon of Escherichia coli is subject to regulation by a phase variation control mechanism in which the pap pilin gene alternates between transcriptionally active (phase-on) and inactive (phase-off) states. Pap phase variation appears to involve differential inhibition of deoxyadenosine methylase (Dam) methylation of two pap GATC sites, GATC1028 and GATC1130, located in the regulatory region upstream of the papBA promoter. DNA from phase-on cells contains an unmethylated adenosine in the GATC1028 site, whereas DNA from phase-off cells contains an unmethylated adenosine in the GATC1130 site. papI and papB are two regulatory genes in the pap operon. Analysis of pap deletion mutants suggests that papI is required for methylation inhibition at the GATC1028 site; however, neither papI nor papB is required for inhibition of methylation at the GATC1130 site. We have identified a chromosomal locus, mbf (methylation-blocking factor), that is required for methylation protection of both the pap GATC1028 and GATC1130 sites. The mbf locus was identified after transposon mTn10 mutagenesis and mapped to 19.6 min on the E. coli chromosome. The effect of transposon mutations within mbf on pap pilin transcription was determined by using a papBAp-lac operon fusion which places lacZ under control of the papBA promoter. E. coli containing mbf::mTn10 and phase-off mbf+ E. coli cells both expressed beta-galactosidase levels about 30-fold lower than the beta-galactosidase level measured for phase-on mbf+ E. coli cells. These results indicated that mbf was necessary for pap pilin transcription and were supported by Northern (RNA) blotting and primer extension analyses. Moreover, transposon insertion within mbf greatly reduced Pap pilus expression. The mbf locus was isolated on a low-copy-number cosmid, pMBF1. Complementation analysis indicated that each of seven mbf::mTn10 mutants isolated contained a transposon insertion within the same gene or operon. The identification of the mbf locus, required for pap transcription, supports the hypothesis that pap phase variation is controlled by a mechanism involving alternation between different methylation states.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird L., Georgopoulos C. Identification, cloning, and characterization of the Escherichia coli sohA gene, a suppressor of the htrA (degP) null phenotype. J Bacteriol. 1990 Mar;172(3):1587–1594. doi: 10.1128/jb.172.3.1587-1594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L. B., Braaten B. A., Low D. A. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 1990 Dec;9(12):4045–4054. doi: 10.1002/j.1460-2075.1990.tb07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L. B., Braaten B. A., White-Ziegler C. A., Rolfson D. H., Low D. A. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 1989 Feb;8(2):613–620. doi: 10.1002/j.1460-2075.1989.tb03416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell. 1988 Jan 29;52(2):197–206. doi: 10.1016/0092-8674(88)90508-9. [DOI] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985 Dec 30;4(13B):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990 Sep 7;62(5):967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Forsman K., Göransson M., Uhlin B. E. Autoregulation and multiple DNA interactions by a transcriptional regulatory protein in E. coli pili biogenesis. EMBO J. 1989 Apr;8(4):1271–1277. doi: 10.1002/j.1460-2075.1989.tb03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier G. E., Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979 Feb 25;254(4):1408–1413. [PubMed] [Google Scholar]
- Göransson M., Forsman K., Uhlin B. E. Regulatory genes in the thermoregulation of Escherichia coli pili gene transcription. Genes Dev. 1989 Jan;3(1):123–130. doi: 10.1101/gad.3.1.123. [DOI] [PubMed] [Google Scholar]
- Karow M., Fayet O., Cegielska A., Ziegelhoffer T., Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33 degrees C in rich media. J Bacteriol. 1991 Jan;173(2):741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Koomey J. M., Gill R. E., Falkow S. Genetic and biochemical analysis of gonococcal IgA1 protease: cloning in Escherichia coli and construction of mutants of gonococci that fail to produce the activity. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7881–7885. doi: 10.1073/pnas.79.24.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D., David V., Lark D., Schoolnik G., Falkow S. Gene clusters governing the production of hemolysin and mannose-resistant hemagglutination are closely linked in Escherichia coli serotype O4 and O6 isolates from urinary tract infections. Infect Immun. 1984 Jan;43(1):353–358. doi: 10.1128/iai.43.1.353-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D., Robinson E. N., Jr, McGee Z. A., Falkow S. The frequency of expression of pyelonephritis-associated pili is under regulatory control. Mol Microbiol. 1987 Nov;1(3):335–346. doi: 10.1111/j.1365-2958.1987.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Lyons S. M., Schendel P. F. Kinetics of methylation in Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):421–423. doi: 10.1128/jb.159.1.421-423.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W., Noyer-Weidner M. Timing and targeting: the biological functions of Dam methylation in E. coli. Cell. 1988 Sep 9;54(6):735–737. doi: 10.1016/s0092-8674(88)90911-7. [DOI] [PubMed] [Google Scholar]
- O'Connor M., Gesteland R. F., Atkins J. F. tRNA hopping: enhancement by an expanded anticodon. EMBO J. 1989 Dec 20;8(13):4315–4323. doi: 10.1002/j.1460-2075.1989.tb08618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Roberts D., Hoopes B. C., McClure W. R., Kleckner N. IS10 transposition is regulated by DNA adenine methylation. Cell. 1985 Nov;43(1):117–130. doi: 10.1016/0092-8674(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Rosen K. M., Lamperti E. D., Villa-Komaroff L. Optimizing the northern blot procedure. Biotechniques. 1990 Apr;8(4):398–403. [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Evidence that adenine methylation influences DNA-protein interactions in Escherichia coli. J Bacteriol. 1985 Oct;164(1):490–493. doi: 10.1128/jb.164.1.490-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- White-Ziegler C. A., Blyn L. B., Braaten B. A., Low D. A. Identification of an Escherichia coli genetic locus involved in thermoregulation of the pap operon. J Bacteriol. 1990 Apr;172(4):1775–1782. doi: 10.1128/jb.172.4.1775-1782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. K., Edlin G. Physical and genetical analysis of bacteriophage T4 generalized transduction. Mol Gen Genet. 1983;192(1-2):241–246. doi: 10.1007/BF00327673. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]