Abstract
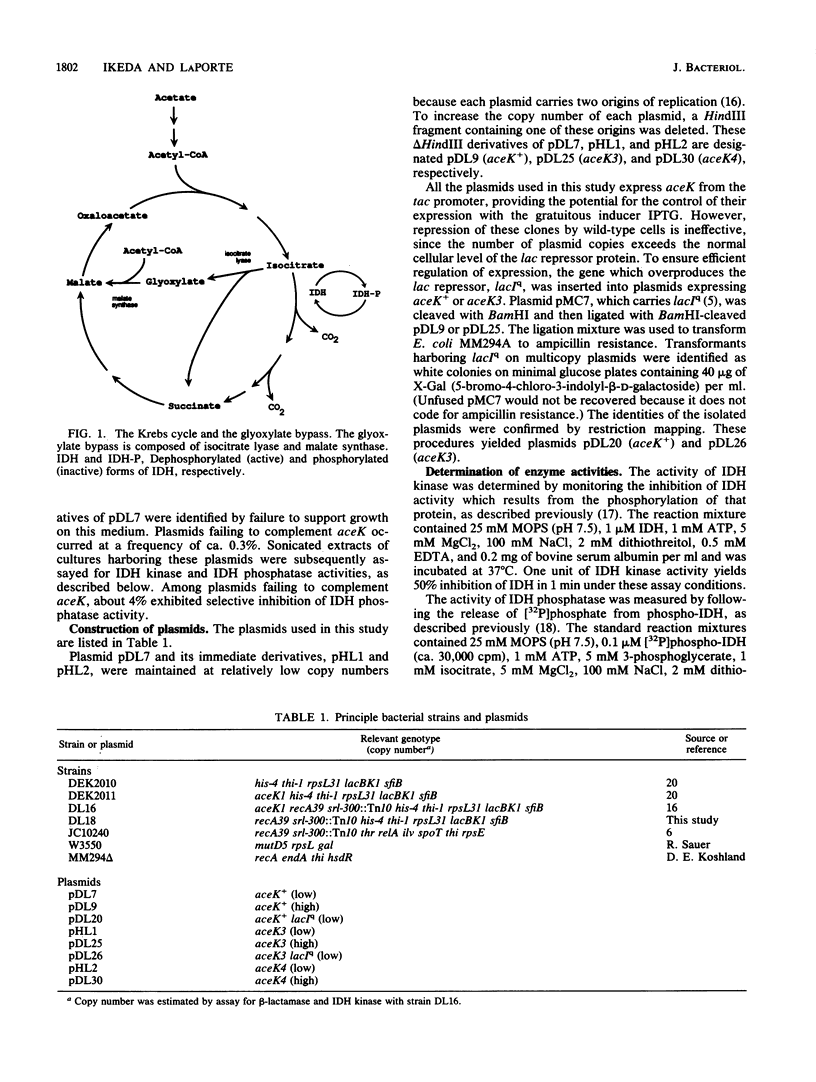
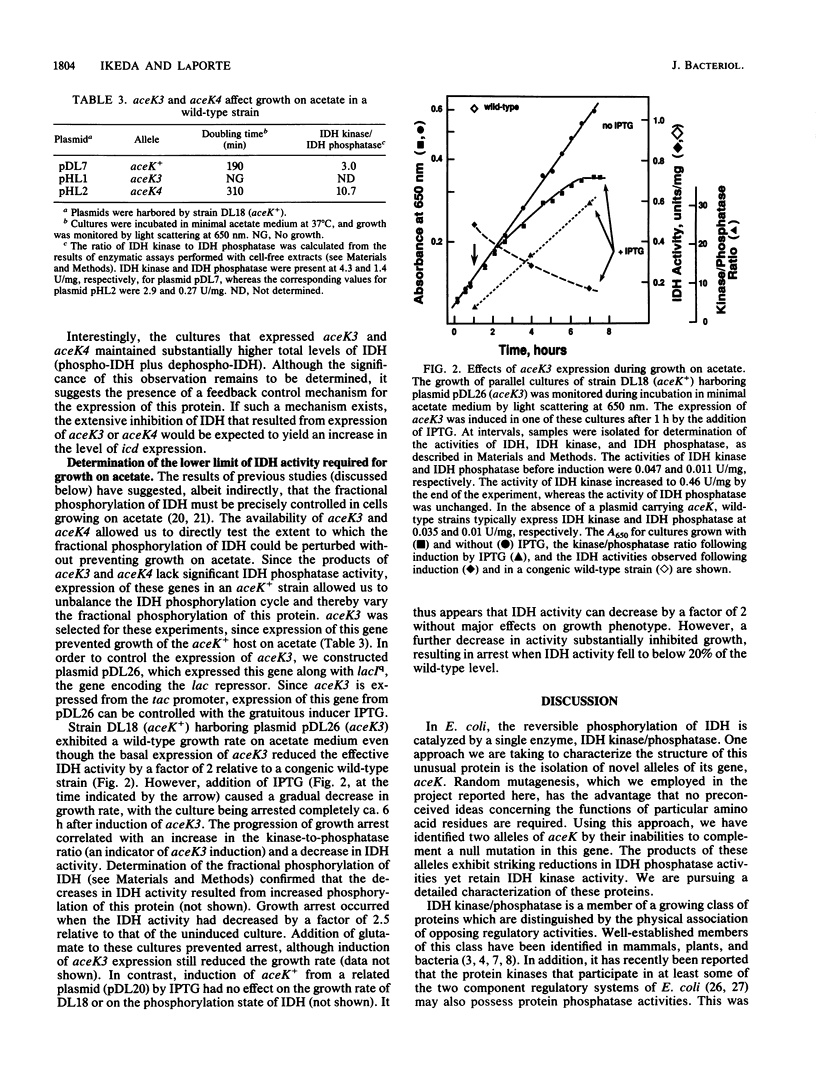
For Escherichia coli, growth on acetate requires the induction of the enzymes of the glyoxylate bypass, isocitrate lyase and malate synthase. The branch point between the glyoxylate bypass and the Krebs cycle is controlled by phosphorylation of isocitrate dehydrogenase (IDH), inhibiting that enzyme's activity and thus forcing isocitrate through the bypass. This phosphorylation cycle is catalyzed by a bifunctional enzyme, IDH kinase/phosphatase, which is encoded by aceK. We have employed random mutagenesis to isolate novel alleles of aceK. These alleles were detected by the loss of ability to complement an aceK null mutation. The products of one class of these alleles retain IDH kinase activity but have suffered reductions in IDH phosphatase activity by factors of 200 to 400. Selective loss of the phosphatase activity also appears to have occurred in vivo, since cells expressing these alleles exhibit phenotypes which are reminiscent of strains lacking IDH; these strains are auxotrophic for glutamate. Assays of cell-free extracts confirmed that this phenotype resulted from nearly quantitative phosphorylation of IDH. The availability of these novel alleles of aceK allowed us to assess the significance of the precise control which is a characteristic of the IDH phosphorylation cycle in vivo. The fractional phosphorylation of IDH was varied by controlled expression of one of the mutant alleles, aceK3, in a wild-type strain. Reduction of IDH activity to 50% of the wild-type level did not adversely affect growth on acetate. However, further reductions inhibited growth, and growth arrest occurred when the IDH activity fell to 15% of the wild-type level. Thus, although wild-type cells maintain a precise effective IDH activity during growth on acetate, this precision is not critical.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. M., Holms W. H. Reversible inactivation of the isocitrate dehydrogenase of Escherichia coli ML308 during growth on acetate. J Gen Microbiol. 1975 Mar;87(1):37–51. doi: 10.1099/00221287-87-1-37. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Activation and inactivation of an enzyme catalyzed by a single, bifunctional protein: a new example and why. Arch Biochem Biophys. 1986 Mar;245(2):297–304. doi: 10.1016/0003-9861(86)90219-5. [DOI] [PubMed] [Google Scholar]
- Caban C. E., Ginsburg A. Glutamine synthetase adenylyltransferase from Escherichia coli: purification and physical and chemical properties. Biochemistry. 1976 Apr 6;15(7):1569–1580. doi: 10.1021/bi00652a030. [DOI] [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):529–530. doi: 10.1128/jb.143.1.529-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J Biol Chem. 1982 Jul 10;257(13):7603–7607. [PubMed] [Google Scholar]
- Garcia E., Rhee S. G. Cascade control of Escherichia coli glutamine synthetase. Purification and properties of PII uridylyltransferase and uridylyl-removing enzyme. J Biol Chem. 1983 Feb 25;258(4):2246–2253. [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Phosphorylation of Isocitrate dehydrogenase of Escherichia coli. Science. 1979 Mar 16;203(4385):1111–1112. doi: 10.1126/science.34215. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Kukora J. S. Nalidixic acd-resistant mutants of Escherichia coli deficient in isocitrate dehydrogenase. J Bacteriol. 1971 Mar;105(3):1224–1226. doi: 10.1128/jb.105.3.1224-1226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Bennett P. M. Regulation of isocitrate dehydrogenase activity in Escherichia coli on adaptation to acetate. J Gen Microbiol. 1971 Jan;65(1):57–68. doi: 10.1099/00221287-65-1-57. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., MADSEN N. B. Synthesis of C4-dicarboxylic acids from acetate by a glyoxylate bypass of the tricarboxylic acid cycle. Biochim Biophys Acta. 1957 Jun;24(3):651–653. doi: 10.1016/0006-3002(57)90268-8. [DOI] [PubMed] [Google Scholar]
- Keener J., Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaPorte D. C., Chung T. A single gene codes for the kinase and phosphatase which regulate isocitrate dehydrogenase. J Biol Chem. 1985 Dec 5;260(28):15291–15297. [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature. 1982 Dec 2;300(5891):458–460. doi: 10.1038/300458a0. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature. 1983 Sep 22;305(5932):286–290. doi: 10.1038/305286a0. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Thorsness P. E., Koshland D. E., Jr Compensatory phosphorylation of isocitrate dehydrogenase. A mechanism for adaptation to the intracellular environment. J Biol Chem. 1985 Sep 5;260(19):10563–10568. [PubMed] [Google Scholar]
- LaPorte D. C., Walsh K., Koshland D. E., Jr The branch point effect. Ultrasensitivity and subsensitivity to metabolic control. J Biol Chem. 1984 Nov 25;259(22):14068–14075. [PubMed] [Google Scholar]
- Laporte D. C., Stueland C. S., Ikeda T. P. Isocitrate dehydrogenase kinase/phosphatase. Biochimie. 1989 Sep-Oct;71(9-10):1051–1057. doi: 10.1016/0300-9084(89)90110-7. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G. The regulatory properties of isocitrate dehydrogenase kinase and isocitrate dehydrogenase phosphatase from Escherichia coli ML308 and the roles of these activities in the control of isocitrate dehydrogenase. Eur J Biochem. 1984 Jun 1;141(2):409–414. doi: 10.1111/j.1432-1033.1984.tb08206.x. [DOI] [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B. T., Ronson C. W., Ausubel F. M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K., Koshland D. E., Jr Determination of flux through the branch point of two metabolic cycles. The tricarboxylic acid cycle and the glyoxylate shunt. J Biol Chem. 1984 Aug 10;259(15):9646–9654. [PubMed] [Google Scholar]



