Abstract
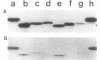
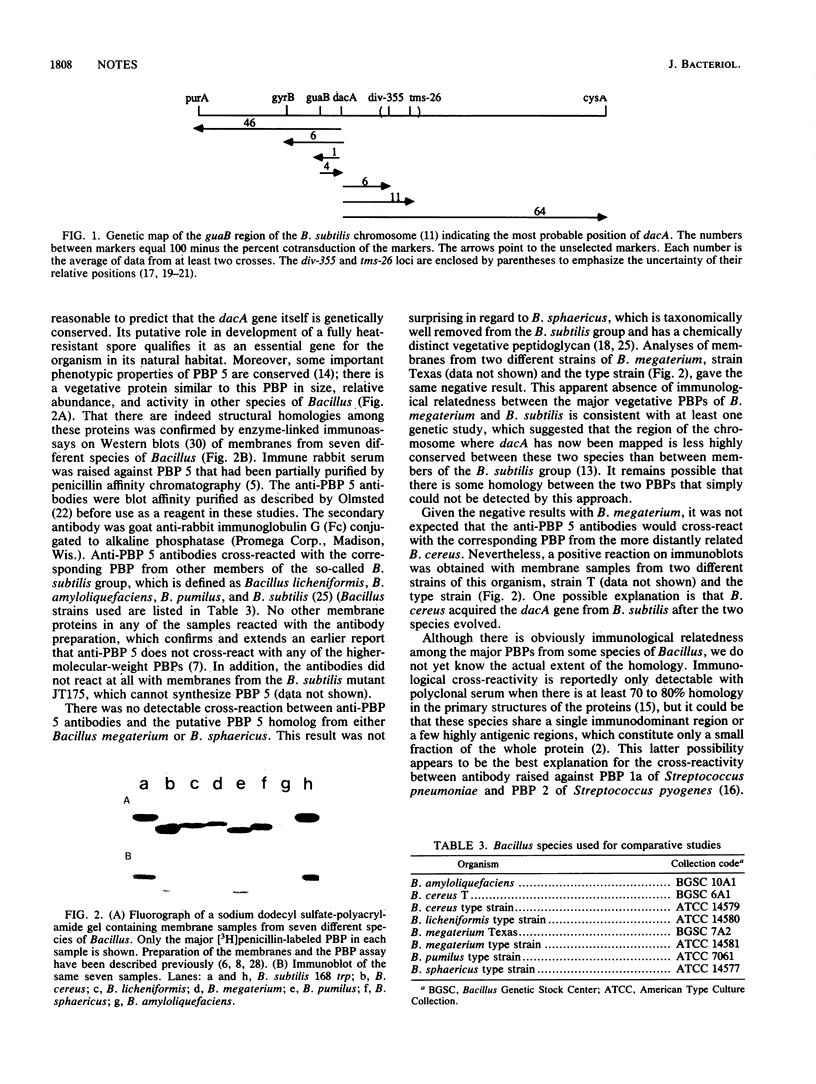
Penicillin-binding protein 5 is the most abundant penicillin-binding protein in the vegetative membranes of Bacillus subtilis and accounts for 95% of the D,D-carboxypeptidase activity of the cell. The structural gene for penicillin-binding protein 5 was mapped to a genetically conserved region near guaB at 0 degrees on the B. subtilis chromosome, and immunoassays revealed that there is conservation of this major penicillin-binding protein among related species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheim N., Prager E. M., Wilson A. C. Immunological prediction of sequence differences among proteins. Chemical comparison of chicken, quail, and phesant lysozymes. J Biol Chem. 1969 Apr 25;244(8):2085–2094. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Covalent affinity chromatography of penicillin-binding components from bacterial membranes. Methods Enzymol. 1974;34:401–405. doi: 10.1016/s0076-6879(74)34046-3. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E., Hsia J., Strominger J. L. Antibody to the D-alanine carboxypeptidase of Bacillus subtilis does not cross-react with other penicillin-binding proteins. J Bacteriol. 1977 Sep;131(3):1008–1010. doi: 10.1128/jb.131.3.1008-1010.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., McCarthy B. J. Genetic and base sequence homologies in bacillus. Genetics. 1969 Jul;62(3):697–710. [PMC free article] [PubMed] [Google Scholar]
- Copeland J. C., Marmur J. Identification of conserved genetic functions in Bacillus by use of temperature-sensitive mutants. Bacteriol Rev. 1968 Dec;32(4 Pt 1):302–312. [PMC free article] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Gasser F., Gasser C. Immunological relationships among lactic dehydrogenases in the genera Lactobacillus and Leuconostoc. J Bacteriol. 1971 Apr;106(1):113–125. doi: 10.1128/jb.106.1.113-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- Nilsson D., Hove-Jensen B., Arnvig K. Primary structure of the tms and prs genes of Bacillus subtilis. Mol Gen Genet. 1989 Sep;218(3):565–571. doi: 10.1007/BF00332425. [DOI] [PubMed] [Google Scholar]
- Nilsson D., Hove-Jensen B. Phosphoribosylpyrophosphate synthetase of Bacillus subtilis. Cloning, characterization and chromosomal mapping of the prs gene. Gene. 1987;53(2-3):247–255. doi: 10.1016/0378-1119(87)90013-8. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Analysis of cytoskeletal structures using blot-purified monospecific antibodies. Methods Enzymol. 1986;134:467–472. doi: 10.1016/0076-6879(86)34112-0. [DOI] [PubMed] [Google Scholar]
- Pai C. H. Genetics of biotin biosynthesis in Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):1–8. doi: 10.1128/jb.121.1.1-8.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G., Goodfellow M., Todd C. A numerical classification of the genus Bacillus. J Gen Microbiol. 1988 Jul;134(7):1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- Sharpe A., Blumberg P. M., Strominger J. L. D-alanine carboxypeptidase and cell wall cross-linking in Bacillus subtilis. J Bacteriol. 1974 Feb;117(2):926–927. doi: 10.1128/jb.117.2.926-927.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell M. O., Buchanan C. E. Changes in penicillin-binding proteins during sporulation of Bacillus subtilis. J Bacteriol. 1983 Mar;153(3):1331–1337. doi: 10.1128/jb.153.3.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Roberts A. N., Johnstone K., Piggot P. J., Winter G., Ellar D. J. Reduced heat resistance of mutant spores after cloning and mutagenesis of the Bacillus subtilis gene encoding penicillin-binding protein 5. J Bacteriol. 1986 Jul;167(1):257–264. doi: 10.1128/jb.167.1.257-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]