Abstract
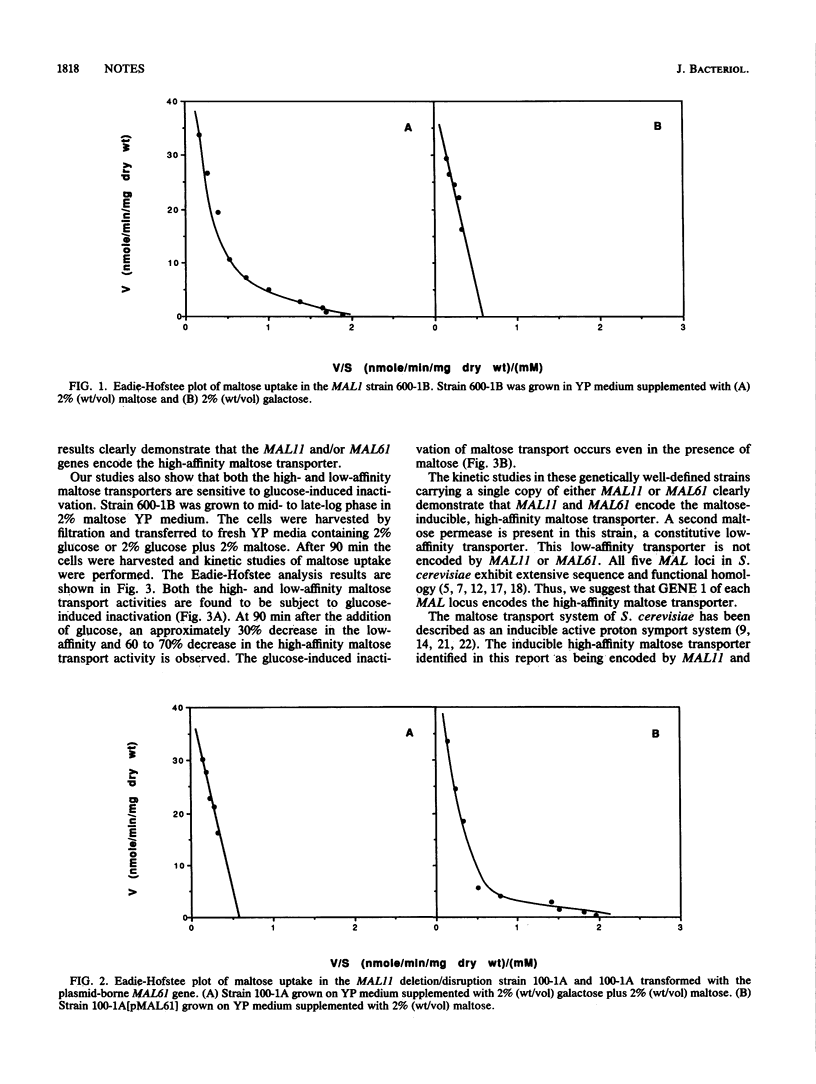
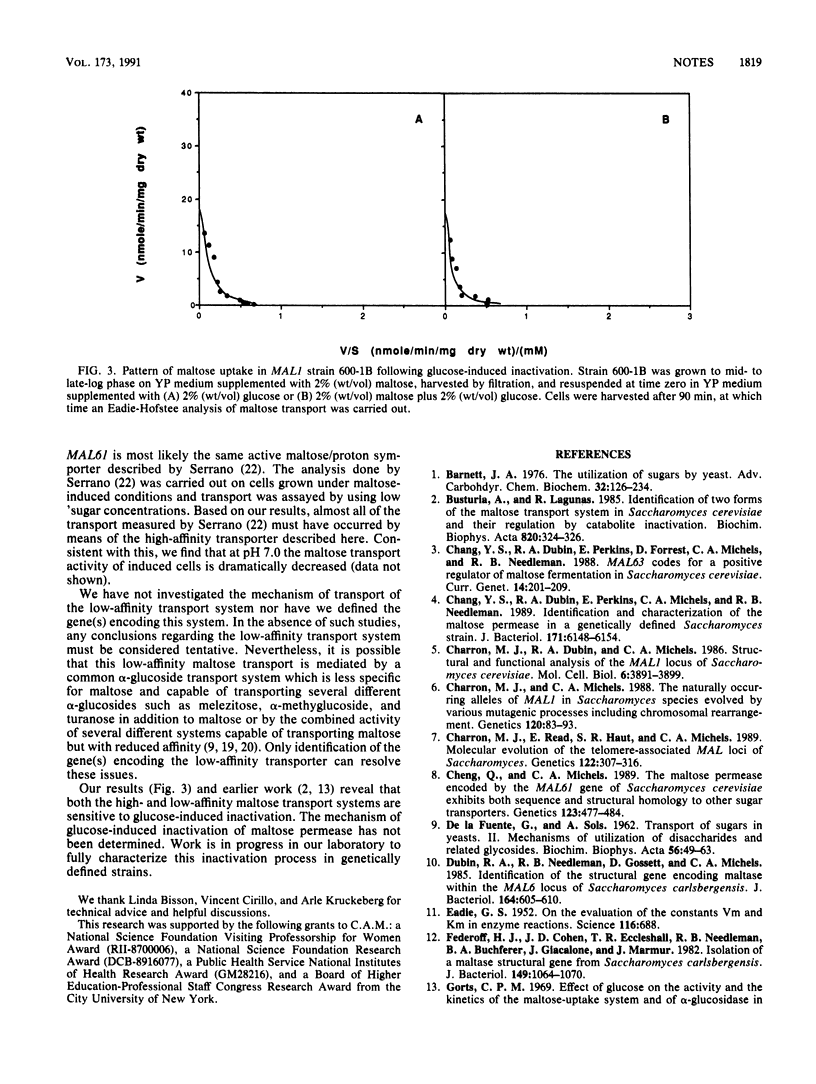
We have investigated the transport of maltose in a genetically defined maltose-fermenting strain of Saccharomyces cerevisiae carrying the MAL1 locus. Two kinetically different systems were identified: a high-affinity transporter with a Km of 4 mM and a low-affinity transporter with a Km of 70 to 80 mM. The high-affinity maltose transporter is maltose inducible and is encoded by the MAL11 (and/or MAL61) gene of the MAL1 (and/or MAL6) locus. The low-affinity maltose transporter is expressed constitutively and is not related to MAL11 and/or MAL61. Both maltose transporters are subject to glucose-induced inactivation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. A. The utilization of sugars by yeasts. Adv Carbohydr Chem Biochem. 1976;32:125–234. doi: 10.1016/s0065-2318(08)60337-6. [DOI] [PubMed] [Google Scholar]
- Chang Y. S., Dubin R. A., Perkins E., Forrest D., Michels C. A., Needleman R. B. MAL63 codes for a positive regulator of maltose fermentation in Saccharomyces cerevisiae. Curr Genet. 1988 Sep;14(3):201–209. doi: 10.1007/BF00376740. [DOI] [PubMed] [Google Scholar]
- Chang Y. S., Dubin R. A., Perkins E., Michels C. A., Needleman R. B. Identification and characterization of the maltose permease in genetically defined Saccharomyces strain. J Bacteriol. 1989 Nov;171(11):6148–6154. doi: 10.1128/jb.171.11.6148-6154.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M. J., Dubin R. A., Michels C. A. Structural and functional analysis of the MAL1 locus of Saccharomyces cerevisiae. Mol Cell Biol. 1986 Nov;6(11):3891–3899. doi: 10.1128/mcb.6.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M. J., Michels C. A. The naturally occurring alleles of MAL1 in Saccharomyces species evolved by various mutagenic processes including chromosomal rearrangement. Genetics. 1988 Sep;120(1):83–93. doi: 10.1093/genetics/120.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M. J., Read E., Haut S. R., Michels C. A. Molecular evolution of the telomere-associated MAL loci of Saccharomyces. Genetics. 1989 Jun;122(2):307–316. doi: 10.1093/genetics/122.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Michels C. A. The maltose permease encoded by the MAL61 gene of Saccharomyces cerevisiae exhibits both sequence and structural homology to other sugar transporters. Genetics. 1989 Nov;123(3):477–484. doi: 10.1093/genetics/123.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA FUENTE G., SOLS A. Transport of sugars in yeasts. II. Mechanisms of utilization of disaccharides and related glycosides. Biochim Biophys Acta. 1962 Jan 1;56:49–62. doi: 10.1016/0006-3002(62)90526-7. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Needleman R. B., Gossett D., Michels C. A. Identification of the structural gene encoding maltase within the MAL6 locus of Saccharomyces carlsbergensis. J Bacteriol. 1985 Nov;164(2):605–610. doi: 10.1128/jb.164.2.605-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EADIE G. S. On the evaluation of the constants Vm and Km in enzyme reactions. Science. 1952 Dec 19;116(3025):688–688. doi: 10.1126/science.116.3025.688. [DOI] [PubMed] [Google Scholar]
- Federoff H. J., Cohen J. D., Eccleshall T. R., Needleman R. B., Buchferer B. A., Giacalone J., Marmur J. Isolation of a maltase structural gene from Saccharomyces carlsbergensis. J Bacteriol. 1982 Mar;149(3):1064–1070. doi: 10.1128/jb.149.3.1064-1070.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS G., THOMPSON C. C. The uptake of nutrients by yeasts. III. The maltose permease of a brewing yeast. Biochim Biophys Acta. 1961 Sep 2;52:176–183. doi: 10.1016/0006-3002(61)90915-5. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Kruckeberg A. L., Bisson L. F. The HXT2 gene of Saccharomyces cerevisiae is required for high-affinity glucose transport. Mol Cell Biol. 1990 Nov;10(11):5903–5913. doi: 10.1128/mcb.10.11.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA H., HALVORSON H. O. UPTAKE OF ALPHA-THIOETHYL D-GLUCOPYRANOSIDE BY SACCHAROMYCES CEREVISIAE. II. GENERAL CHARACTERISTICS OF AN ACTIVE TRANSPORT SYSTEM. Biochim Biophys Acta. 1964 Mar 16;82:547–555. doi: 10.1016/0304-4165(64)90446-5. [DOI] [PubMed] [Google Scholar]
- Perkins E. L., Needleman R. B. MAL64c is a global regulator of alpha-glucoside fermentation: identification of a new gene involved in melezitose fermentation. Curr Genet. 1988 May;13(5):369–375. doi: 10.1007/BF00365657. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. J., HALVORSON H. O. The components of maltozymase in yeast, and their behavior during deadaptation. J Bacteriol. 1957 Feb;73(2):186–198. doi: 10.1128/jb.73.2.186-198.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Energy requirements for maltose transport in yeast. Eur J Biochem. 1977 Oct 17;80(1):97–102. doi: 10.1111/j.1432-1033.1977.tb11861.x. [DOI] [PubMed] [Google Scholar]



