Abstract
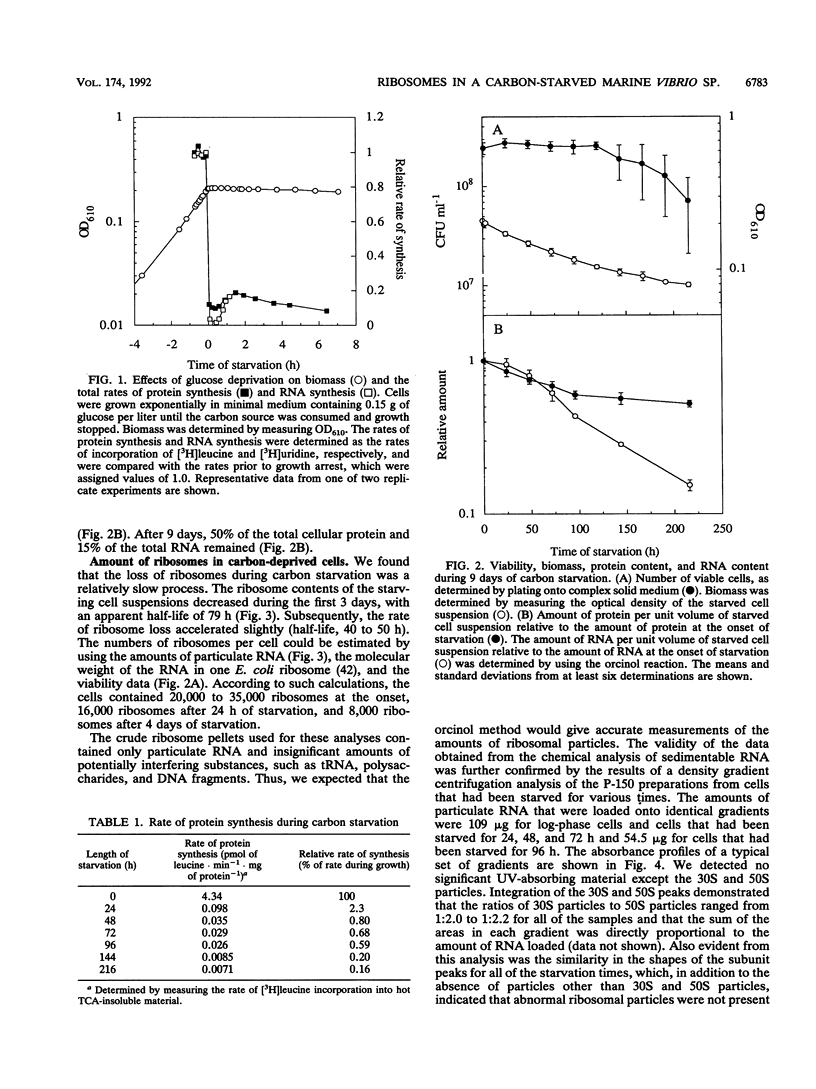
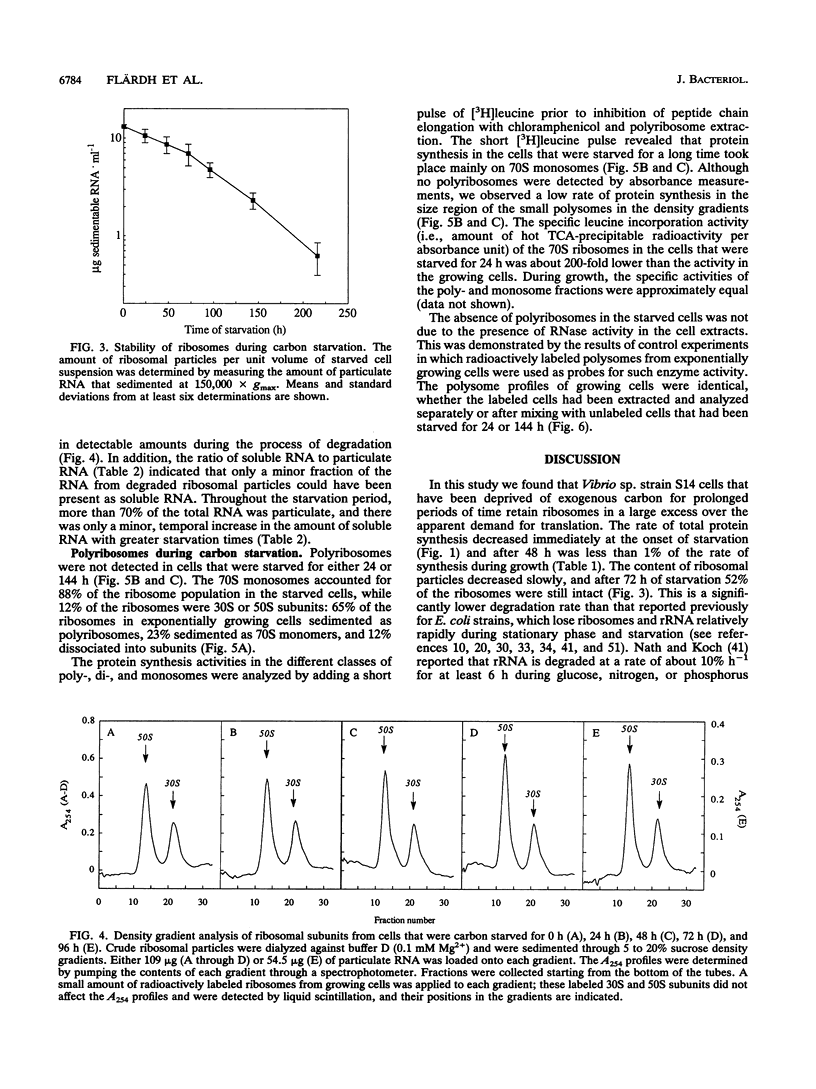
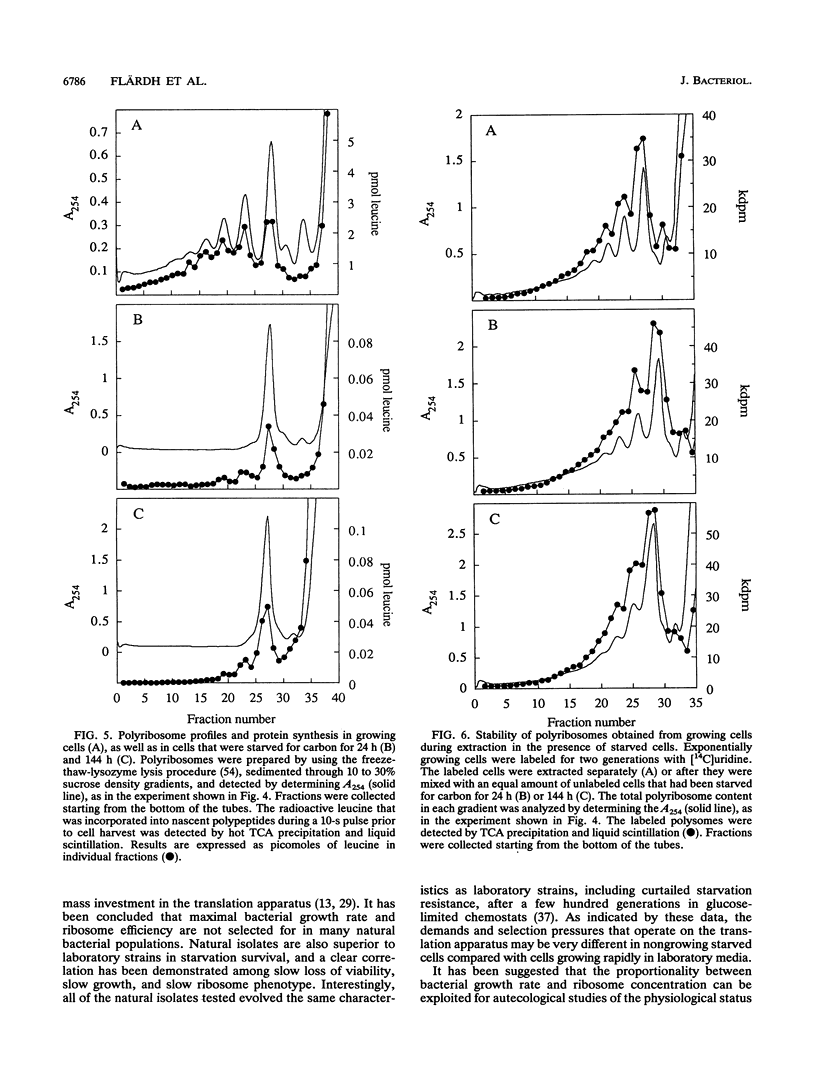
Carbon starvation induces the development of a starvation- and stress-resistant cell state in marine Vibrio sp. strain S14 (CCUG 15956). The starved cells remain highly responsive to nutrients during prolonged starvation and exhibit instantaneous severalfold increases in the rates of protein synthesis and RNA synthesis when substrate is added. In order to elucidate the physiological basis for the survival of cells that are starved for a long time, as well as the capacity of these cells for rapid and efficient recovery, we analyzed the ribosome content of carbon-starved Vibrio sp. strain S14 cells. By using direct chemical measurements of the amounts of ribosomal particles in carbon-starved cultures, we demonstrated that ribosomes were lost relatively slowly (half life, 79 h) and that they existed in large excess over the apparent demand for protein synthesis. After 24 h of starvation the total rate of protein synthesis was 2.3% of the rate during growth, and after 3 days this rate was 0.7% of the rate during growth; the relative amounts of ribosomal particles at these times were 81 and 52%, respectively. The ribosome population consisted of 90% 70S monoribosomes, and no polyribosomes were detected in the starved cells. The 70S monoribosomes were responsible for the bulk of the protein synthesis during carbon starvation; some activity was also detected in the polyribosome size region on sucrose density gradients. We suggest that nongrowing carbon-starved Vibrio sp. strain S14 cells possess an excess protein synthesis capacity, which may be essential for their ability to immediately initiate an upshift program when substrate is added.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertson N. H., Nyström T., Kjelleberg S. Exoprotease Activity of Two Marine Bacteria during Starvation. Appl Environ Microbiol. 1990 Jan;56(1):218–223. doi: 10.1128/aem.56.1.218-223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson N. H., Nyström T., Kjelleberg S. Starvation-induced modulations in binding protein-dependent glucose transport by the marine Vibrio sp. S14. FEMS Microbiol Lett. 1990 Jul;58(2):205–209. doi: 10.1111/j.1574-6968.1990.tb13979.x. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Luger S. M., Tai P. C. Role of ribosome degradation in the death of starved Escherichia coli cells. J Bacteriol. 1986 May;166(2):439–445. doi: 10.1128/jb.166.2.439-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch M., Lane D., Stackebrandt E. Towards a phylogeny of the genus Vibrio based on 16S rRNA sequences. Int J Syst Bacteriol. 1992 Jan;42(1):58–63. doi: 10.1099/00207713-42-1-58. [DOI] [PubMed] [Google Scholar]
- Dresden M. H., Hoagland M. B. Polyribosomes of Escherichia coli. Breakdown during glucose starvation. J Biol Chem. 1967 Mar 10;242(5):1065–1068. [PubMed] [Google Scholar]
- Ehrenberg M., Kurland C. G. Costs of accuracy determined by a maximal growth rate constraint. Q Rev Biophys. 1984 Feb;17(1):45–82. doi: 10.1017/s0033583500005254. [DOI] [PubMed] [Google Scholar]
- Gottschal J. C. Substrate capturing and growth in various ecosystems. Soc Appl Bacteriol Symp Ser. 1992;21:39S–48S. doi: 10.1111/j.1365-2672.1992.tb03623.x. [DOI] [PubMed] [Google Scholar]
- Groat R. G., Schultz J. E., Zychlinsky E., Bockman A., Matin A. Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol. 1986 Nov;168(2):486–493. doi: 10.1128/jb.168.2.486-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Auger E. A., Matin A. Role of RpoH, a heat shock regulator protein, in Escherichia coli carbon starvation protein synthesis and survival. J Bacteriol. 1991 Mar;173(6):1992–1996. doi: 10.1128/jb.173.6.1992-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Chaisson S. A., Matin A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol. 1990 May;172(5):2779–2781. doi: 10.1128/jb.172.5.2779-2781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURLAND C. G., NOMURA M., WATSON J. D. The physical properties of the chloromycetin particles. J Mol Biol. 1962 May;4:388–394. doi: 10.1016/s0022-2836(62)80019-9. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Apirion D. The fate of ribosomes in Escherichia coli cells starved for a carbon source. J Biol Chem. 1975 Mar 10;250(5):1854–1863. [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Kramer J. G., Singleton F. L. Variations in rRNA content of marine Vibrio spp. during starvation-survival and recovery. Appl Environ Microbiol. 1992 Jan;58(1):201–207. doi: 10.1128/aem.58.1.201-207.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Mizuno D. Ribosome degradation and the degradation products in starved Escherichia coli. I. Comparison of the degradation rate and of the nucleotide pool between Escherichia coli B and Q-13 strains in phosphate deficiency. Biochim Biophys Acta. 1970 Jan 21;199(1):159–165. [PubMed] [Google Scholar]
- Maruyama H., Ono M., Mizuno D. Ribosome degradation and the degradation products in starved Escherichia coli. 3. Ribosomal RNA degradation during the complete deprivation of nutrients. Biochim Biophys Acta. 1970 Jan 21;199(1):176–183. doi: 10.1016/0005-2787(70)90706-9. [DOI] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. II. Strain differences in the degradation of protein and nucleic acid resulting from starvation. J Biol Chem. 1971 Nov 25;246(22):6956–6967. [PubMed] [Google Scholar]
- Nyström T., Albertson N., Kjelleberg S. Synthesis of membrane and periplasmic proteins during starvation of a marine Vibrio sp. J Gen Microbiol. 1988 Jun;134(6):1645–1651. doi: 10.1099/00221287-134-6-1645. [DOI] [PubMed] [Google Scholar]
- Nyström T., Flärdh K., Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990 Dec;172(12):7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Olsson R. M., Kjelleberg S. Survival, stress resistance, and alterations in protein expression in the marine vibrio sp. strain S14 during starvation for different individual nutrients. Appl Environ Microbiol. 1992 Jan;58(1):55–65. doi: 10.1128/aem.58.1.55-65.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura S., Maruyama H. B., Yanagita T. Ribosome degradation and degradation products in starved Escherichia coli. VI. Prolonged culture during glucose starvation. J Biochem. 1973 May;73(5):915–922. doi: 10.1093/oxfordjournals.jbchem.a130174. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Ron E. Z., Kohler R. E., Davis B. D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science. 1966 Sep 2;153(3740):1119–1120. doi: 10.1126/science.153.3740.1119. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Latter G. I., Matin A. Differential regulation by cyclic AMP of starvation protein synthesis in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3903–3909. doi: 10.1128/jb.170.9.3903-3909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegele D. A., Kolter R. Life after log. J Bacteriol. 1992 Jan;174(2):345–348. doi: 10.1128/jb.174.2.345-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Almirón M., Kolter R. surA, an Escherichia coli gene essential for survival in stationary phase. J Bacteriol. 1990 Aug;172(8):4339–4347. doi: 10.1128/jb.172.8.4339-4347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A., Yamazaki Y., Fujita N., Ishihama A. Structure and probable genetic location of a "ribosome modulation factor" associated with 100S ribosomes in stationary-phase Escherichia coli cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2657–2661. doi: 10.1073/pnas.87.7.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]


