Abstract
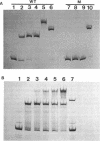
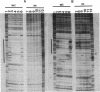
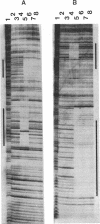
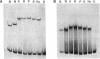
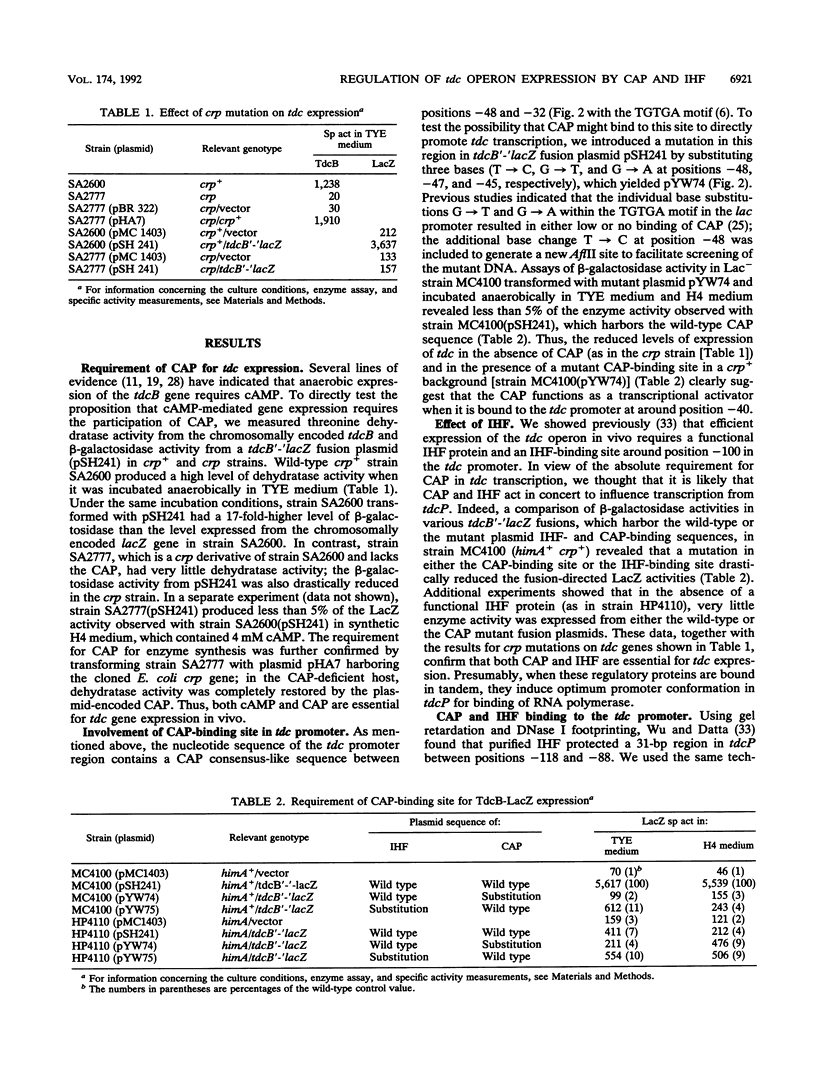
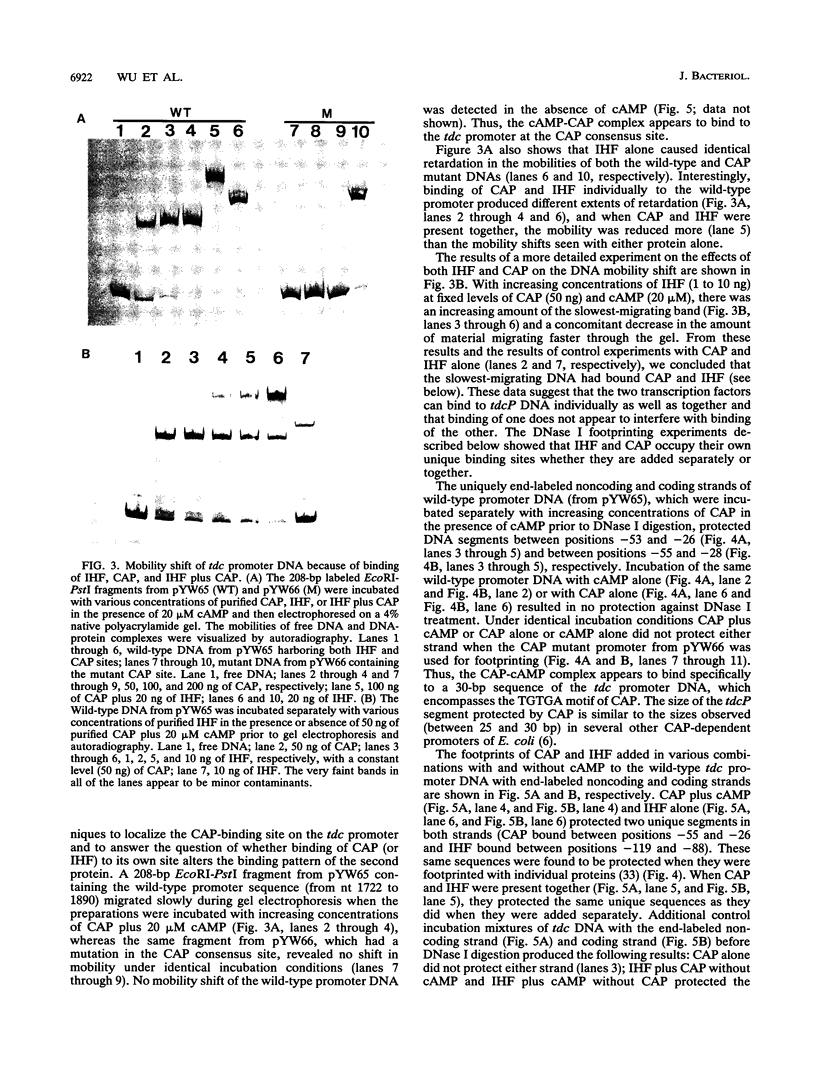
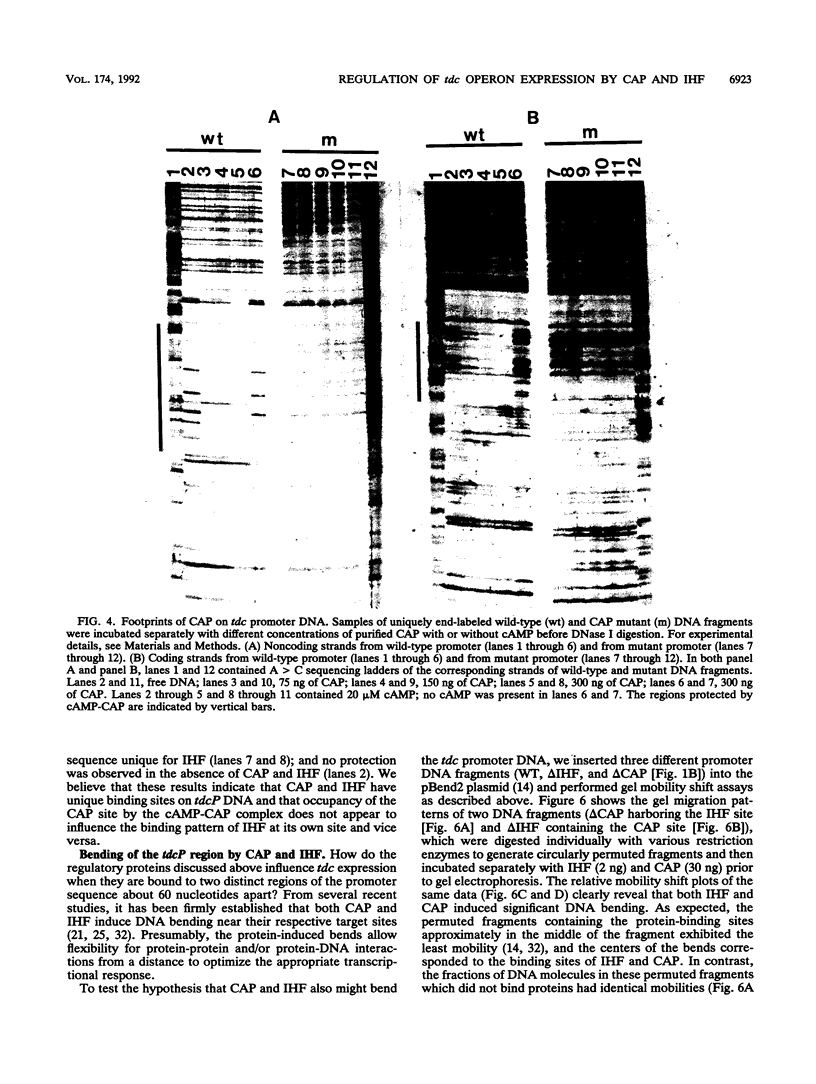
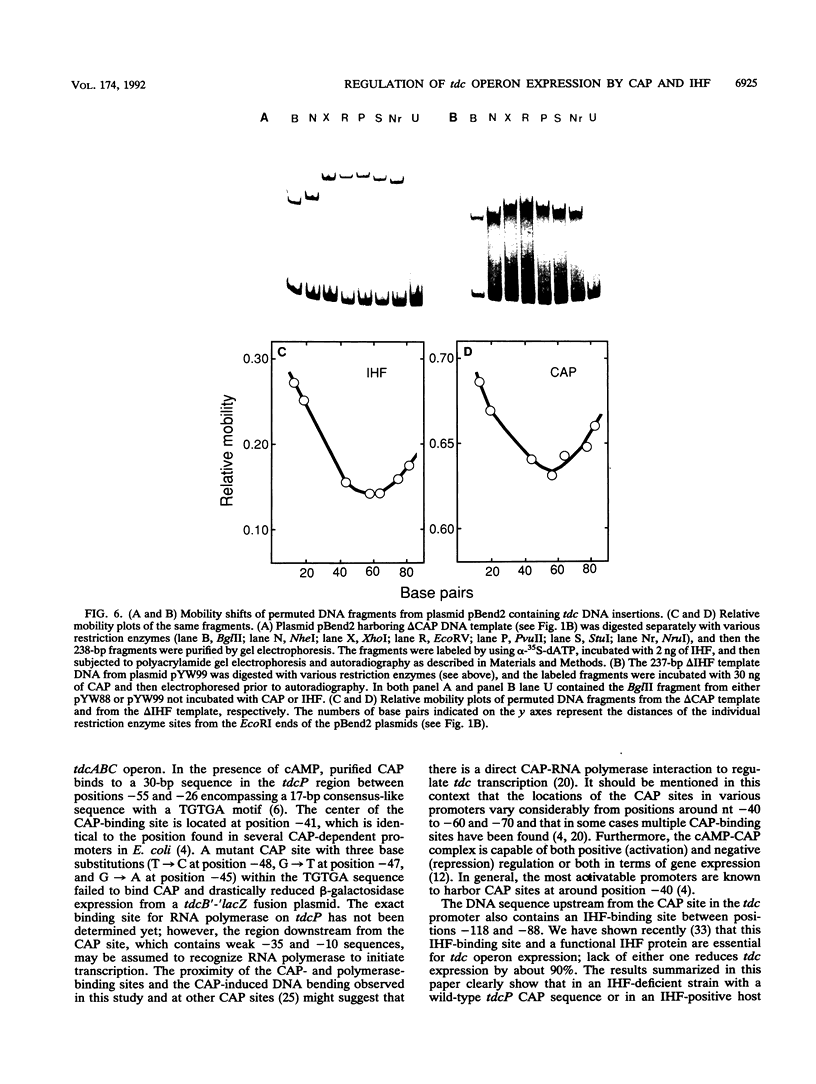
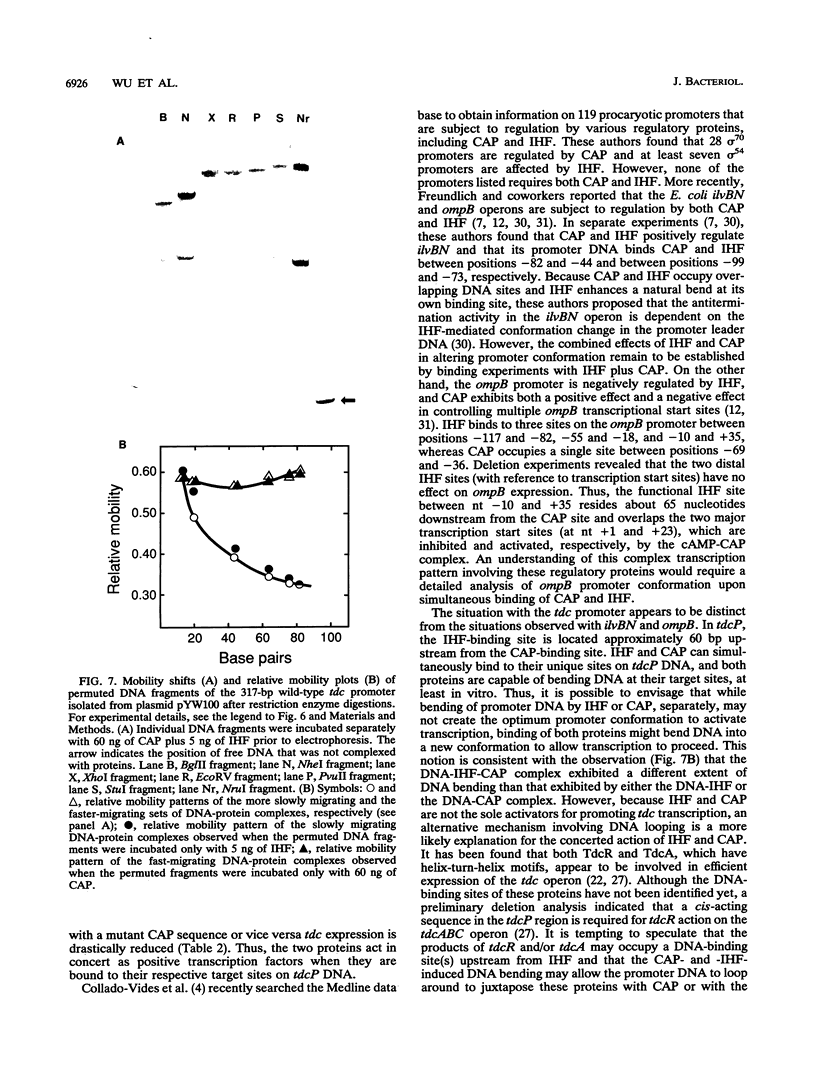
Anaerobic expression of the tdcABC operon of Escherichia coli requires cyclic AMP and the catabolite gene activator protein (CAP). Purified CAP binds to a 30-bp sequence in the tdc promoter between positions -55 and -26, and a mutant CAP site with base substitutions at positions -48, -47, and -45 failed to bind CAP and also drastically reduced the beta-galactosidase expression from a tdcB'-'lacZ fusion plasmid. Recently, we showed that efficient expression of the tdc operon also requires a functional integration host factor (IHF) and an IHF-binding site in the tdc promoter between positions -118 and -88. The levels of beta-galactosidase activity from the tdcB'-'lacZ fusion plasmids were also reduced in an IHF-deficient strain with the wild-type or mutant plasmid CAP sequence. In vitro footprinting experiments revealed that CAP and IHF occupy their specific binding sites on tdc DNA when they are present separately or together. These regulatory proteins also induced significant bending of the tdc promoter DNA. Our results suggest that CAP and IHF act in concert as positive transcription factors for tdc operon expression in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Fujimoto S., Ozaki N. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res. 1982 Feb 25;10(4):1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone T., Wilcox G. A rapid high-yield purification procedure for the cyclic adenosine 3',5'-monophosphate receptor protein from Escherichia coli. Biochim Biophys Acta. 1978 Jul 17;541(4):528–534. doi: 10.1016/0304-4165(78)90162-9. [DOI] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Goss T. J., Omnaas J. R., Patil R. V. Covalent structure of biodegradative threonine dehydratase of Escherichia coli: homology with other dehydratases. Proc Natl Acad Sci U S A. 1987 Jan;84(2):393–397. doi: 10.1073/pnas.84.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss T. J., Datta P. Molecular cloning and expression of the biodegradative threonine dehydratase gene (tdc) of Escherichia coli K12. Mol Gen Genet. 1985;201(2):308–314. doi: 10.1007/BF00425676. [DOI] [PubMed] [Google Scholar]
- Goss T. J., Schweizer H. P., Datta P. Molecular characterization of the tdc operon of Escherichia coli K-12. J Bacteriol. 1988 Nov;170(11):5352–5359. doi: 10.1128/jb.170.11.5352-5359.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W., Schleif R. F. Regulation of the Escherichia coli L-arabinose operon studied by gel electrophoresis DNA binding assay. J Mol Biol. 1984 Sep 25;178(3):611–628. doi: 10.1016/0022-2836(84)90241-9. [DOI] [PubMed] [Google Scholar]
- Hobert E. H., Datta P. Synthesis of biodegradative threonine dehydratase in Escherichia coli: role of amino acids, electron acceptors, and certain intermediary metabolites. J Bacteriol. 1983 Aug;155(2):586–592. doi: 10.1128/jb.155.2.586-592.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Tsui P., Freundlich M. Positive and negative control of ompB transcription in Escherichia coli by cyclic AMP and the cyclic AMP receptor protein. J Bacteriol. 1992 Feb;174(3):664–670. doi: 10.1128/jb.174.3.664-670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. M., Fried M. G. Co-operative interactions between the catabolite gene activator protein and the lac repressor at the lactose promoter. J Mol Biol. 1990 Jul 20;214(2):381–396. doi: 10.1016/0022-2836(90)90188-R. [DOI] [PubMed] [Google Scholar]
- Kim J., Zwieb C., Wu C., Adhya S. Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene. 1989 Dec 21;85(1):15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A. Purification and properties of the Escherichia coli protein factor required for lambda integrative recombination. J Biol Chem. 1981 Sep 10;256(17):9246–9253. [PubMed] [Google Scholar]
- Phillips A. T., Egan R. M., Lewis B. Control of biodegradative threonine dehydratase inducibility by cyclic AMP in energy-restricted Escherichia coli. J Bacteriol. 1978 Sep;135(3):828–840. doi: 10.1128/jb.135.3.828-840.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S. Catabolite gene activator protein activation of lac transcription. J Bacteriol. 1992 Feb;174(3):655–658. doi: 10.1128/jb.174.3.655-658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Schweizer H. P., Datta P. Identification and DNA sequence of tdcR, a positive regulatory gene of the tdc operon of Escherichia coli. Mol Gen Genet. 1989 Sep;218(3):516–522. doi: 10.1007/BF00332418. [DOI] [PubMed] [Google Scholar]
- Schweizer H. P., Datta P. The complete nucleotide sequence of the tdc region of Escherichia coli. Nucleic Acids Res. 1989 May 25;17(10):3994–3994. doi: 10.1093/nar/17.10.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuta Y., Hayaishi O. Regulation of biodegradative threonine deaminase. Curr Top Cell Regul. 1976;11:99–146. doi: 10.1016/b978-0-12-152811-9.50010-9. [DOI] [PubMed] [Google Scholar]
- Sumantran V. N., Schweizer H. P., Datta P. A novel membrane-associated threonine permease encoded by the tdcC gene of Escherichia coli. J Bacteriol. 1990 Aug;172(8):4288–4294. doi: 10.1128/jb.172.8.4288-4294.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui P., Freundlich M. Integration host factor bends the DNA in the Escherichia coli ilvBN promoter region. Mol Gen Genet. 1990 Sep;223(2):349–352. doi: 10.1007/BF00265076. [DOI] [PubMed] [Google Scholar]
- Tsui P., Huang L., Freundlich M. Integration host factor binds specifically to multiple sites in the ompB promoter of Escherichia coli and inhibits transcription. J Bacteriol. 1991 Sep;173(18):5800–5807. doi: 10.1128/jb.173.18.5800-5807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
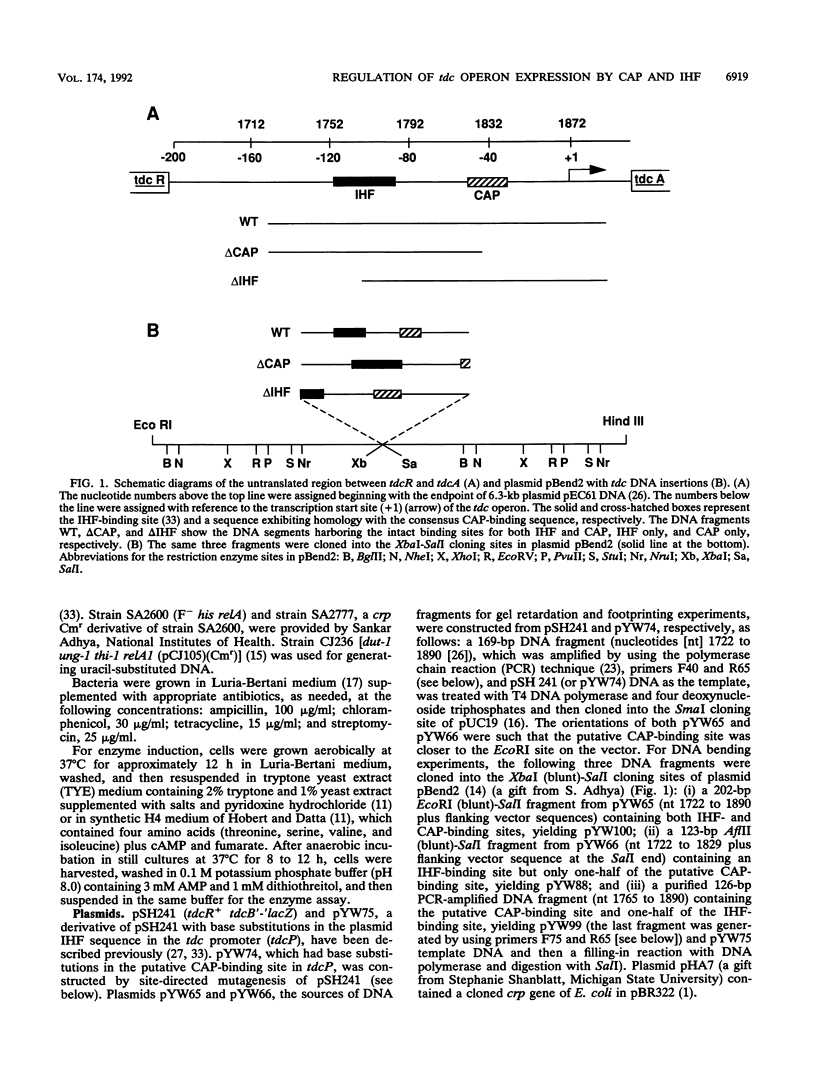
- Wu Y. F., Datta P. Integration host factor is required for positive regulation of the tdc operon of Escherichia coli. J Bacteriol. 1992 Jan;174(1):233–240. doi: 10.1128/jb.174.1.233-240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]