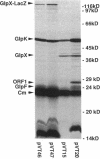
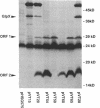
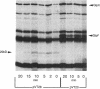
Abstract
We have identified a new gene, glpX, belonging to the glp regulon of Escherichia coli, located directly downstream of the glpK gene. The transcription of glpX is inducible with glycerol and sn-glycerol-3-phosphate and is constitutive in a glpR mutant. glpX is the third gene in the glpFKX operon. The function of GlpX remains unknown. GlpX has an apparent molecular weight of 40,000 on sodium dodecyl sulfate-polyacrylamide gels. In addition to determining the E. coli glpX sequence, we also sequenced the corresponding glpFKX region originating from Shigella flexneri, which after transfer into E. coli was instrumental in elucidating the function of glpF in glycerol transport (D. P. Richey and E. C. C. Lin, J. Bacteriol. 112:784-790, 1972). Sequencing of the glpFKX region of this hybrid strain revealed an amber mutation instead of the tryptophan 215 codon in glpF. The most striking difference between the E. coli and S. flexneri DNA was found directly behind glpK, where two repetitive (REP) sequences were present in S. flexneri, but not in the E. coli sequence. The presence or absence of these REP sequences had no effect on transport or on growth on glycerol. Not including the REP sequence-containing region, only 1.1% of a total of 2,167 bp sequenced was different in the two sequences. Comparison of the sequence with those in the EMBL data library revealed a 99% identity between the last third of glpX and the first part of a gene called mvrA. We show that the cloned mvrA gene (M. Morimyo, J. Bacteriol. 170:2136-2142, 1988) originated from the 88-min region of the Escherichia coli chromosome and not, as reported, from the 7-min region and that the gene product identified as MvrA is in fact encoded by a gene distal to glpX.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASNIS R. E., BRODIE A. F. A glycerol dehydrogenase from Escherichia coli. J Biol Chem. 1953 Jul;203(1):153–159. [PubMed] [Google Scholar]
- Alefounder P. R., Perham R. N. Identification, molecular cloning and sequence analysis of a gene cluster encoding the class II fructose 1,6-bisphosphate aldolase, 3-phosphoglycerate kinase and a putative second glyceraldehyde 3-phosphate dehydrogenase of Escherichia coli. Mol Microbiol. 1989 Jun;3(6):723–732. doi: 10.1111/j.1365-2958.1989.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bulawa C. E., Raetz C. R. Isolation and characterization of Escherichia coli strains defective in CDP-diglyceride hydrolase. J Biol Chem. 1984 Sep 25;259(18):11257–11264. [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Dimri G. P., Rudd K. E., Morgan M. K., Bayat H., Ames G. F. Physical mapping of repetitive extragenic palindromic sequences in Escherichia coli and phylogenetic distribution among Escherichia coli strains and other enteric bacteria. J Bacteriol. 1992 Jul;174(14):4583–4593. doi: 10.1128/jb.174.14.4583-4593.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Stoehr P., Rice P., Omond R., Cameron G. New services of the EMBL Data Library. Nucleic Acids Res. 1990 Aug 11;18(15):4319–4323. doi: 10.1093/nar/18.15.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Bachellier S., Perrin S., Perrin D., Grimont P. A., Grimont F., Hofnung M. Palindromic unit highly repetitive DNA sequences exhibit species specificity within Enterobacteriaceae. Res Microbiol. 1990 Nov-Dec;141(9):1103–1116. doi: 10.1016/0923-2508(90)90084-4. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L. Maltose chemoreceptor of Escherichia coli. J Bacteriol. 1975 Apr;122(1):206–214. doi: 10.1128/jb.122.1.206-214.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. W., Engel R., Tropp B. E. Correlation of 3,4-dihydroxybutyl 1-phosphonate resistance with a defect in cardiolipin synthesis in Escherichia coli. J Bacteriol. 1984 Mar;157(3):846–856. doi: 10.1128/jb.157.3.846-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. J., Dekker E. E. Identity of Escherichia coli D-1-amino-2-propanol:NAD+ oxidoreductase with E. coli glycerol dehydrogenase but not with Neisseria gonorrhoeae 1,2-propanediol:NAD+ oxidoreductase. J Bacteriol. 1985 Apr;162(1):170–175. doi: 10.1128/jb.162.1.170-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R., Corwin L. M. Mutation in Shigella flexneri resulting in loss of ability to penetrate HeLa cells and loss of glycerol kinase activity. Infect Immun. 1974 May;9(5):916–923. doi: 10.1128/iai.9.5.916-923.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Zhang Y. H., Rieger M., Minter M., Hsu B., Ooi B. G., Koeuth T., McCabe E. R. Mutational analysis of the Escherichia coli glpFK region with Tn5 mutagenesis and the polymerase chain reaction. J Bacteriol. 1990 Oct;172(10):6129–6134. doi: 10.1128/jb.172.10.6129-6134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Latshaw S. P., Steup M., Gerbling K. P. Partial amino acid sequence of fructose-1,6-bisphosphatase from the blue-green algae Synechococcus leopoliensis. Protein Seq Data Anal. 1989 Aug;2(5):391–393. [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Morimyo M. Isolation and characterization of methyl viologen-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1988 May;170(5):2136–2142. doi: 10.1128/jb.170.5.2136-2142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 Jun 12;17(11):4378–4378. [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew D. W., Ma D. P., Conrad C. A., Johnson J. R. Escherichia coli glycerol kinase. Cloning and sequencing of the glpK gene and the primary structure of the enzyme. J Biol Chem. 1988 Jan 5;263(1):135–139. [PubMed] [Google Scholar]
- Pluschke G., Hirota Y., Overath P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 1978 Jul 25;253(14):5048–5055. [PubMed] [Google Scholar]
- Pluschke G., Overath P. Function of phospholipids in Escherichia coli. Influence of changes in polar head group composition on the lipid phase transition and characterization of a mutant containing only saturated phospholipid acyl chains. J Biol Chem. 1981 Apr 10;256(7):3207–3212. [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W., Larson T. J. Repressor for the sn-glycerol-3-phosphate regulon of Escherichia coli K-12: cloning of the glpR gene and identification of its product. J Bacteriol. 1985 Feb;161(2):563–566. doi: 10.1128/jb.161.2.563-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Sweet G., Larson T. J. Physical and genetic structure of the glpD-malT interval of the Escherichia coli K-12 chromosome. Identification of two new structural genes of the glp-regulon. Mol Gen Genet. 1986 Mar;202(3):488–492. doi: 10.1007/BF00333282. [DOI] [PubMed] [Google Scholar]
- St Martin E. J., Freedberg W. B., Lin E. C. Kinase replacement by a dehydrogenase for Escherichia coli glycerol utilization. J Bacteriol. 1977 Sep;131(3):1026–1028. doi: 10.1128/jb.131.3.1026-1028.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Tang C. T., Ruch F. E., Jr, Lin C. C. Purification and properties of a nicotinamide adenine dinucleotide-linked dehydrogenase that serves an Escherichia coli mutant for glycerol catabolism. J Bacteriol. 1979 Oct;140(1):182–187. doi: 10.1128/jb.140.1.182-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986 Sep 5;191(1):39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Weissenborn D. L., Wittekindt N., Larson T. J. Structure and regulation of the glpFK operon encoding glycerol diffusion facilitator and glycerol kinase of Escherichia coli K-12. J Biol Chem. 1992 Mar 25;267(9):6122–6131. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]