Abstract
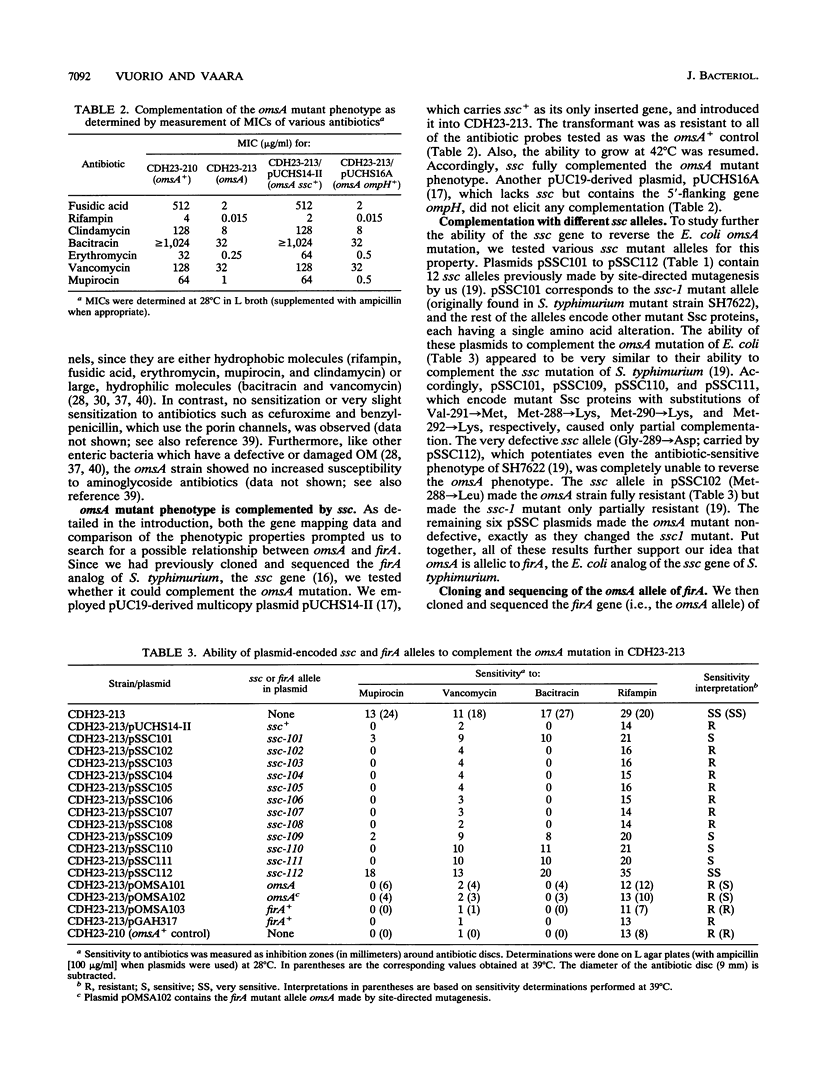
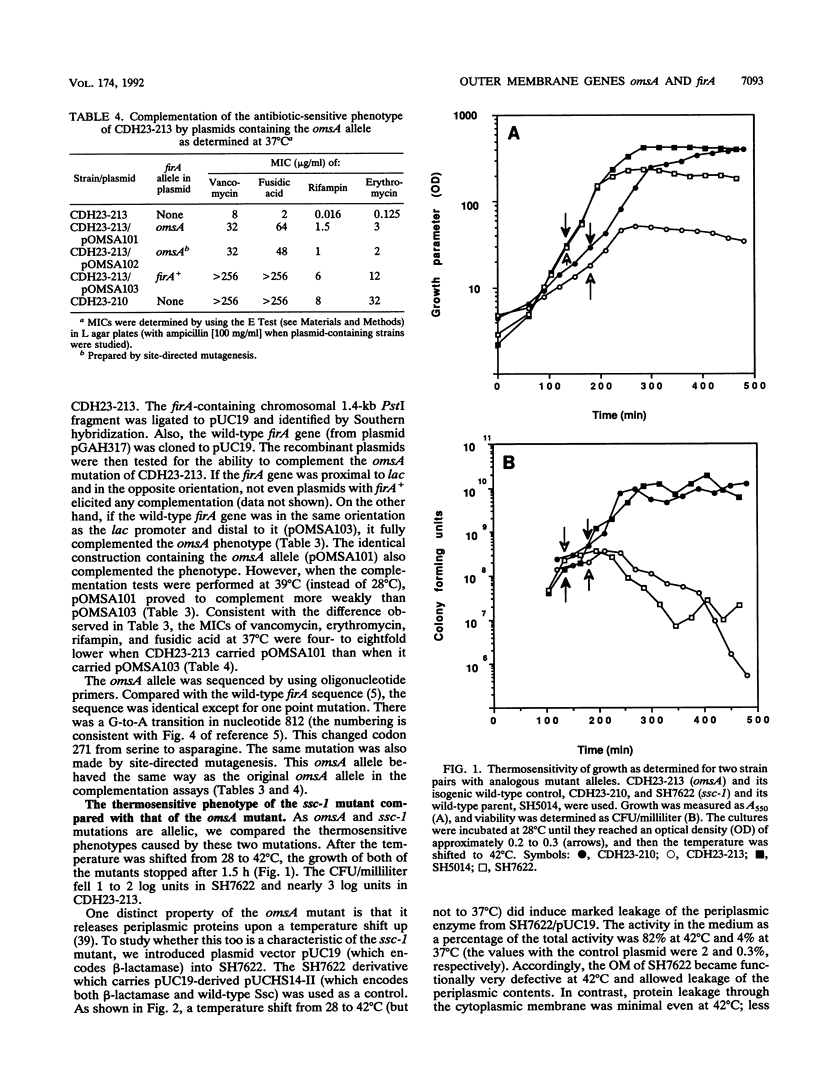
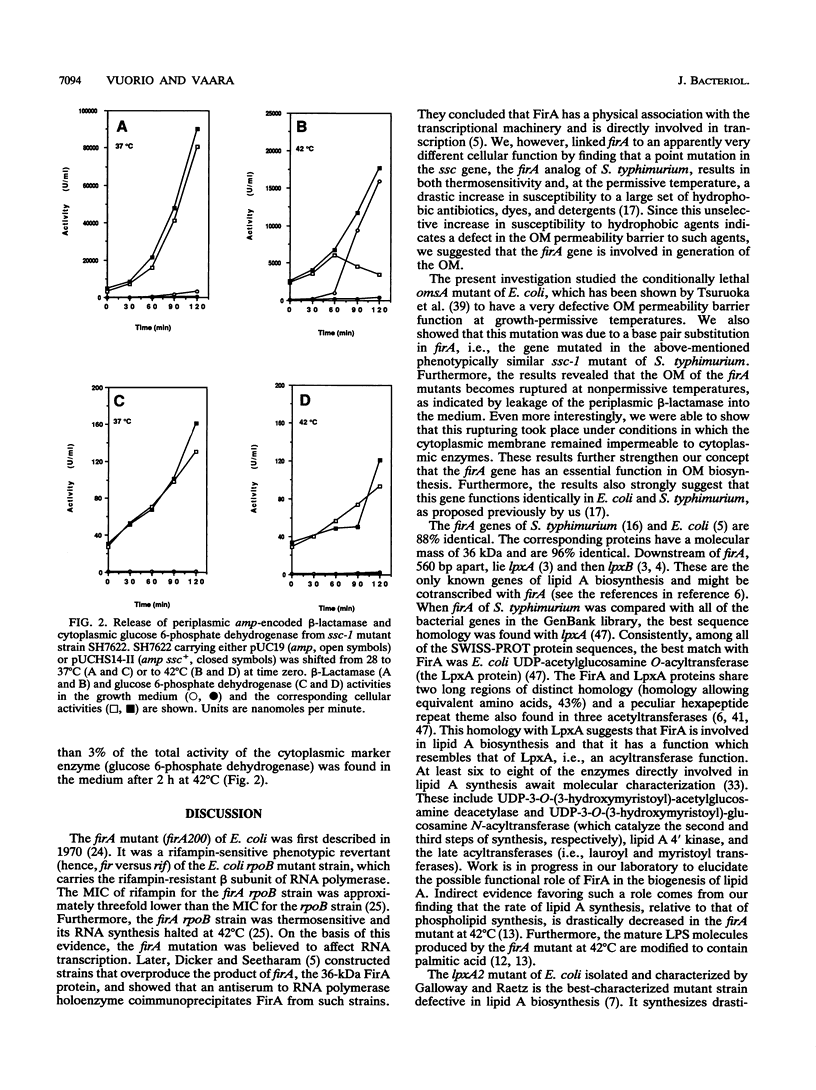
We have previously identified the gene (the ssc gene) defective in the thermosensitive and antibiotic-supersusceptible outer membrane permeability mutant SS-C of Salmonella typhimurium and shown that this gene is analogous to the Escherichia coli gene firA (L. Hirvas, P. Koski, and M. Vaara, EMBO J. 10:1017-1023, 1991). Others have tentatively implicated firA in a different function, mRNA synthesis. Here we report that the defect in the thermosensitive outer membrane omsA mutant of E. coli (T. Tsuruoka, M. Ito, S. Tomioka, A. Hirata, and M. Matsuhashi, J. Bacteriol. 170:5229-5235, 1988) is due to a mutation in firA; this mutation changed codon 271 from serine to asparagine. The omsA-induced phenotype was completely reverted by plasmids containing wild-type firA or ssc. Plasmids carrying the omsA allele, or an identical mutant allele prepared by localized mutagenesis, under the control of lac elicited partial complementation. Transcomplementation studies with plasmids carrying various mutant alleles of the S. typhimurium gene indicated that the ability of these plasmids to complement the omsA mutation was similar to their ability to complement the ssc mutation. The antibiotic-supersusceptible phenotype of the omsA mutant closely resembled that of the ssc mutant, i.e., the omsA mutant was supersusceptible to hydrophobic antibiotics and large-peptide antibiotics against which the intact outer membrane is an effective permeability barrier. As previously demonstrated with the omsA mutant, the outer membrane of the ssc mutant became selectively ruptured after incubation for 1 h at the growth-nonpermitting temperature; 82% of the periplasmic beta-lactamase and less than 3% of the cytoplasmic marker enzyme were released into the medium. All of these findings are consistent with our concept that firA is an essential gene involved in generation of the outer membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alting-Mees M. A., Short J. M. pBluescript II: gene mapping vectors. Nucleic Acids Res. 1989 Nov 25;17(22):9494–9494. doi: 10.1093/nar/17.22.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Raetz C. R. First committed step of lipid A biosynthesis in Escherichia coli: sequence of the lpxA gene. J Bacteriol. 1988 Mar;170(3):1268–1274. doi: 10.1128/jb.170.3.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell D. N., Reznikoff W. S., Raetz C. R. Nucleotide sequence of the Escherichia coli gene for lipid A disaccharide synthase. J Bacteriol. 1987 Dec;169(12):5727–5734. doi: 10.1128/jb.169.12.5727-5734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker I. B., Seetharam S. Cloning and nucleotide sequence of the firA gene and the firA200(Ts) allele from Escherichia coli. J Bacteriol. 1991 Jan;173(1):334–344. doi: 10.1128/jb.173.1.334-344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker I. B., Seetharam S. What is known about the structure and function of the Escherichia coli protein FirA? Mol Microbiol. 1992 Apr;6(7):817–823. doi: 10.1111/j.1365-2958.1992.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Raetz C. R. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem. 1990 Apr 15;265(11):6394–6402. [PubMed] [Google Scholar]
- Goldman R., Kohlbrenner W., Lartey P., Pernet A. Antibacterial agents specifically inhibiting lipopolysaccharide synthesis. Nature. 1987 Sep 10;329(6135):162–164. doi: 10.1038/329162a0. [DOI] [PubMed] [Google Scholar]
- Hammond S. M., Claesson A., Jansson A. M., Larsson L. G., Pring B. G., Town C. M., Ekström B. A new class of synthetic antibacterials acting on lipopolysaccharide biosynthesis. 1987 Jun 25-Jul 1Nature. 327(6124):730–732. doi: 10.1038/327730a0. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Hirvas L., Tuominen J., Vaara M. Preferential synthesis of heptaacyl lipopolysaccharide by the ssc permeability mutant of Salmonella typhimurium. Eur J Biochem. 1992 Mar 15;204(3):1101–1106. doi: 10.1111/j.1432-1033.1992.tb16734.x. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Vaara M., Sukupolvi S., Rhen M., Saarela S., Zähringer U., Mäkelä P. H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989 Nov 20;185(3):541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Hirvas L., Coleman J., Koski P., Vaara M. Bacterial 'histone-like protein I' (HLP-I) is an outer membrane constituent? FEBS Lett. 1990 Mar 12;262(1):123–126. doi: 10.1016/0014-5793(90)80169-j. [DOI] [PubMed] [Google Scholar]
- Hirvas L., Koski P., Vaara M. Identification and sequence analysis of the gene mutated in the conditionally lethal outer membrane permeability mutant SS-C of Salmonella typhimurium. EMBO J. 1991 Apr;10(4):1017–1023. doi: 10.1002/j.1460-2075.1991.tb08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvas L., Koski P., Vaara M. Primary structure and expression of the Ssc-protein of Salmonella typhimurium. Biochem Biophys Res Commun. 1990 Nov 30;173(1):53–59. doi: 10.1016/s0006-291x(05)81020-4. [DOI] [PubMed] [Google Scholar]
- Hirvas L., Koski P., Vaara M. The ompH gene of Yersinia enterocolitica: cloning, sequencing, expression, and comparison with known enterobacterial ompH sequences. J Bacteriol. 1991 Feb;173(3):1223–1229. doi: 10.1128/jb.173.3.1223-1229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvas L., Vaara M. Effect of Ssc protein mutations on the outer membrane permeability barrier function in Salmonella typhimurium: a study using ssc mutant alleles made by site-directed mutagenesis. FEMS Microbiol Lett. 1992 Jan 15;69(3):289–294. doi: 10.1016/0378-1097(92)90662-8. [DOI] [PubMed] [Google Scholar]
- Holck A., Kleppe K. Cloning and sequencing of the gene for the DNA-binding 17K protein of Escherichia coli. Gene. 1988 Jul 15;67(1):117–124. doi: 10.1016/0378-1119(88)90014-5. [DOI] [PubMed] [Google Scholar]
- Koski P., Hirvas L., Vaara M. Complete sequence of the ompH gene encoding the 16-kDa cationic outer membrane protein of Salmonella typhimurium. Gene. 1990 Mar 30;88(1):117–120. doi: 10.1016/0378-1119(90)90068-3. [DOI] [PubMed] [Google Scholar]
- Koski P., Rhen M., Kantele J., Vaara M. Isolation, cloning, and primary structure of a cationic 16-kDa outer membrane protein of Salmonella typhimurium. J Biol Chem. 1989 Nov 15;264(32):18973–18980. [PubMed] [Google Scholar]
- Lathe R., Buc H., Lecocq J. P., Bautz E. K. Prokaryotic histone-like protein interacting with RNA polymerase. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3548–3552. doi: 10.1073/pnas.77.6.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Fine-structure mapping of the firA gene, a locus involved in the phenotypic expression of rifampin resistance in Escherichia. J Bacteriol. 1977 Sep;131(3):1033–1036. doi: 10.1128/jb.131.3.1033-1036.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M. Salmonella typhimurium and Escherichia coli mutants with increased outer membrane permeability to hydrophobic compounds. Biochim Biophys Acta. 1989 Dec 6;988(3):377–387. doi: 10.1016/0304-4157(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Tsuruoka T., Ito M., Tomioka S., Hirata A., Matsuhashi M. Thermosensitive omsA mutation of Escherichia coli that causes thermoregulated release of periplasmic proteins. J Bacteriol. 1988 Nov;170(11):5229–5235. doi: 10.1128/jb.170.11.5229-5235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992 Sep;56(3):395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Plachy W. Z., Nikaido H. Partitioning of hydrophobic probes into lipopolysaccharide bilayers. Biochim Biophys Acta. 1990 May 9;1024(1):152–158. doi: 10.1016/0005-2736(90)90218-d. [DOI] [PubMed] [Google Scholar]
- Vuorio R., Hirvas L., Raybourne R. B., Yu D. T., Vaara M. The nucleotide and deduced amino acid sequence of the cationic 19 kDa outer membrane protein OmpH of Yersinia pseudotuberculosis. Biochim Biophys Acta. 1991 Dec 2;1129(1):124–126. doi: 10.1016/0167-4781(91)90226-c. [DOI] [PubMed] [Google Scholar]
- Vuorio R., Hirvas L., Vaara M. The Ssc protein of enteric bacteria has significant homology to the acyltransferase Lpxa of lipid A biosynthesis, and to three acetyltransferases. FEBS Lett. 1991 Nov 4;292(1-2):90–94. doi: 10.1016/0014-5793(91)80841-p. [DOI] [PubMed] [Google Scholar]
- Vuorio R., Vaara M. The lipid A biosynthesis mutation lpxA2 of Escherichia coli results in drastic antibiotic supersusceptibility. Antimicrob Agents Chemother. 1992 Apr;36(4):826–829. doi: 10.1128/aac.36.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



