Abstract
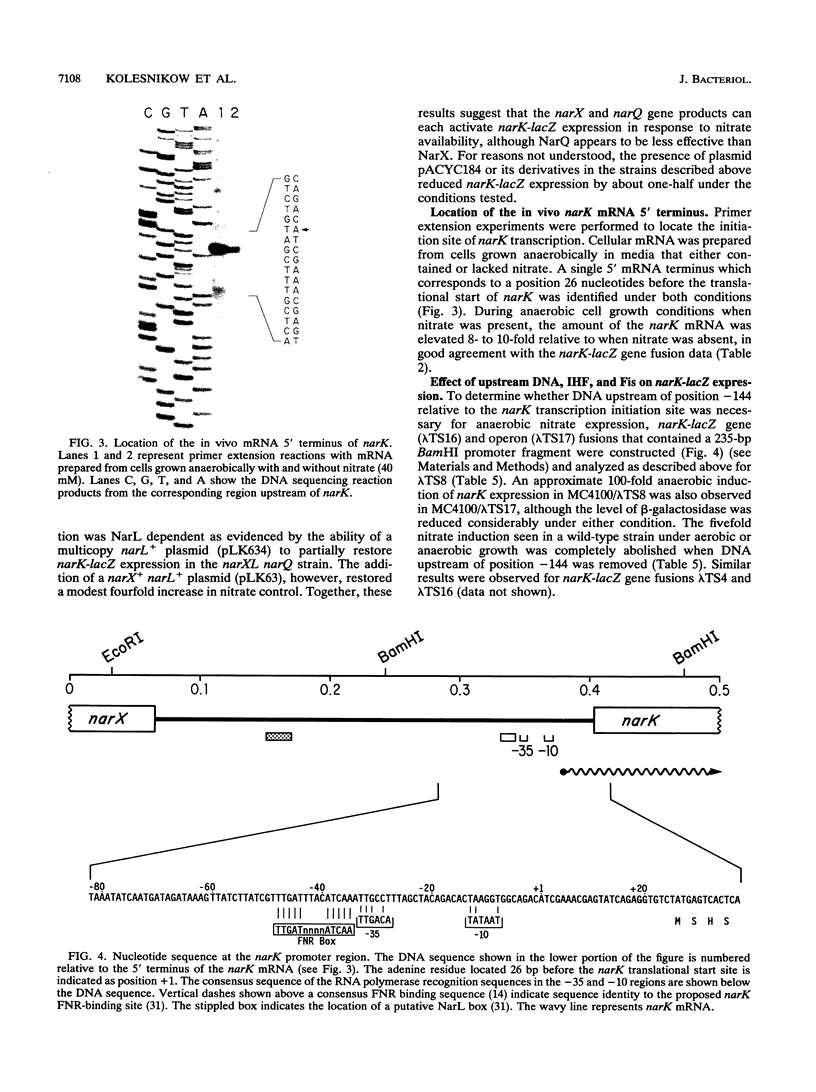
The regulation of the narK gene in Escherichia coli was studied by constructing narK-lacZ gene and operon fusions and analyzing their expression in various mutant strains in response to changes in cell growth conditions. Expression of narK-lacZ was induced 110-fold by a shift to anaerobic growth and a further 8-fold by the presence of nitrate. The fnr gene product mediates this anaerobic response, while nitrate control is mediated by the narL, narX, and narQ gene products. The narX and narQ gene products were shown to sense nitrate independently of one another and could each activate narK expression in a NarL-dependent manner. We provide the first evidence that NarL and FNR interact to ensure optimal expression of narK. IHF and Fis proteins are also required for full activation of narK expression, and their roles in DNA bending are discussed. Finally, the availability of molybdate and iron ions is necessary for optimal narK expression, whereas the availability of nitrite is not. Although the role of the narK gene product in cell metabolism remains uncertain, the pattern of narK gene expression is consistent with a proposed role of NarK in nitrate uptake by the cell for nitrate-linked electron transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang R. C., Cavicchioli R., Gunsalus R. P. Identification and characterization of narQ, a second nitrate sensor for nitrate-dependent gene regulation in Escherichia coli. Mol Microbiol. 1992 Jul;6(14):1913–1923. doi: 10.1111/j.1365-2958.1992.tb01364.x. [DOI] [PubMed] [Google Scholar]
- Cotter P. A., Chepuri V., Gennis R. B., Gunsalus R. P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990 Nov;172(11):6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. A., Gunsalus R. P. Oxygen, nitrate, and molybdenum regulation of dmsABC gene expression in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3817–3823. doi: 10.1128/jb.171.7.3817-3823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- DeMoss J. A., Hsu P. Y. NarK enhances nitrate uptake and nitrite excretion in Escherichia coli. J Bacteriol. 1991 Jun;173(11):3303–3310. doi: 10.1128/jb.173.11.3303-3310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. R., Li S. F., DeMoss J. A. Upstream sequence elements required for NarL-mediated activation of transcription from the narGHJI promoter of Escherichia coli. J Biol Chem. 1992 Jul 15;267(20):14122–14128. [PubMed] [Google Scholar]
- Egan S. M., Stewart V. Nitrate regulation of anaerobic respiratory gene expression in narX deletion mutants of Escherichia coli K-12. J Bacteriol. 1990 Sep;172(9):5020–5029. doi: 10.1128/jb.172.9.5020-5029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Grove C. L., Gunsalus R. P. Regulation of the aroH operon of Escherichia coli by the tryptophan repressor. J Bacteriol. 1987 May;169(5):2158–2164. doi: 10.1128/jb.169.5.2158-2164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Kalman L. V., Stewart R. R. Nucleotide sequence of the narL gene that is involved in global regulation of nitrate controlled respiratory genes of Escherichia coli. Nucleic Acids Res. 1989 Mar 11;17(5):1965–1975. doi: 10.1093/nar/17.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton C. M., Aldea M., Washburn B. K., Babitzke P., Kushner S. R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Molybdenum effector of fumarate reductase repression and nitrate reductase induction in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3720–3725. doi: 10.1128/jb.169.8.3720-3725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987 Jul;169(7):3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman L. V., Gunsalus R. P. Identification of a second gene involved in global regulation of fumarate reductase and other nitrate-controlled genes for anaerobic respiration in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3810–3816. doi: 10.1128/jb.171.7.3810-3816.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman L. V., Gunsalus R. P. Nitrate- and molybdenum-independent signal transduction mutations in narX that alter regulation of anaerobic respiratory genes in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7049–7056. doi: 10.1128/jb.172.12.7049-7056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman L. V., Gunsalus R. P. The frdR gene of Escherichia coli globally regulates several operons involved in anaerobic growth in response to nitrate. J Bacteriol. 1988 Feb;170(2):623–629. doi: 10.1128/jb.170.2.623-629.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrewa D., Granzin J., Koch C., Choe H. W., Raghunathan S., Wolf W., Labahn J., Kahmann R., Saenger W. Three-dimensional structure of the E. coli DNA-binding protein FIS. Nature. 1991 Jan 10;349(6305):178–180. doi: 10.1038/349178a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Murakawa G. J., Kwan C., Yamashita J., Nierlich D. P. Transcription and decay of the lac messenger: role of an intergenic terminator. J Bacteriol. 1991 Jan;173(1):28–36. doi: 10.1128/jb.173.1.28-36.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus F., Hantke K., Unden G. Iron content and FNR-dependent gene regulation in Escherichia coli. FEMS Microbiol Lett. 1991 Dec 1;68(3):319–323. doi: 10.1016/0378-1097(91)90376-l. [DOI] [PubMed] [Google Scholar]
- Nohno T., Noji S., Taniguchi S., Saito T. The narX and narL genes encoding the nitrate-sensing regulators of Escherichia coli are homologous to a family of prokaryotic two-component regulatory genes. Nucleic Acids Res. 1989 Apr 25;17(8):2947–2957. doi: 10.1093/nar/17.8.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Nohno T., Saito T., Taniguchi S. The narK gene product participates in nitrate transport induced in Escherichia coli nitrate-respiring cells. FEBS Lett. 1989 Jul 31;252(1-2):139–143. doi: 10.1016/0014-5793(89)80906-8. [DOI] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990 Aug;6(4):399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Spiro S., Roberts R. E., Guest J. R. FNR-dependent repression of the ndh gene of Escherichia coli and metal ion requirement for FNR-regulated gene expression. Mol Microbiol. 1989 May;3(5):601–608. doi: 10.1111/j.1365-2958.1989.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Parales J., Jr Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J Bacteriol. 1988 Apr;170(4):1589–1597. doi: 10.1128/jb.170.4.1589-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Parales J., Jr, Merkel S. M. Structure of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J Bacteriol. 1989 Apr;171(4):2229–2234. doi: 10.1128/jb.171.4.2229-2234.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J Bacteriol. 1982 Sep;151(3):1320–1325. doi: 10.1128/jb.151.3.1320-1325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. F., Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. doi: 10.1093/nar/16.20.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]