Abstract
d-myo-inositol 1,2,3,4,5,6-hexakisphosphate (InsP6), formed via complex pathways of inositol phosphate metabolism, composes the main bulk of inositol polyphosphates in the cell. Relatively little is known regarding possible biological functions for InsP6. We now show that InsP6 can modulate insulin exocytosis in permeabilized insulin-secreting cells. Concentrations of InsP6 above 20 μM stimulated insulin secretion at basal Ca2+-concentration (30 nM) and primed Ca2+-induced exocytosis (10 μM), both effects being due to activation of protein kinase C. Our results suggest that InsP6 can play an important modulatory role in the regulation of processes such as exocytosis in insulin-secreting cells. The specific role for InsP6 can then be to recruit secretory granules to the site of exocytosis.
Keywords: insulin exocytosis, HIT T15 cells
Highly phosphorylated inositol phosphates are the major inositol phosphates present in the cell (1). Specifically, the intracellular concentration of d-myo-inositol 1,2,3,4,5,6-hexakisphosphate (InsP6) reaches levels corresponding to 40–60 μM in several cell types, including insulin-secreting cells (2–4). In some cell types the concentration of these inositol polyphosphates can be rapidly altered upon cell stimulation (5, 6).
Inositol polyphosphates may be involved in the control of several cellular functions (1), one of which is regulation of vesicle trafficking, processes of endocytosis and vesicle recycling. d-myo-inositol 1,3,4,5-tetrakisphosphate, d-myo-inositol 1,3,4,5,6-pentakisphosphate (InsP5) and InsP6 bind to the C2B domain of synaptotagmin (7), a protein believed to be a main Ca2+-sensor in regulated exocytosis in neurons. The injection of inositol polyphosphates into squid giant synapse preterminal caused blocking of synaptic transmission, due to binding of injected compounds to the C2B domain of synaptotagmin (8). Inositol polyphosphates binding to clathrin assembly proteins, adaptor proteins AP-2 (9), and AP-3 (10) inhibit clathrin assembly, suggesting the influence of the compounds upon vesicle trafficking (10).
The aim of the present study was to evaluate a possible role for InsP6 in the regulation of insulin exocytosis.
MATERIALS AND METHODS
Materials.
InsP5 and InsP6 were from Calbiochem–Novabiochem. Streptolysin O was obtained from Difco. All other reagents were from Sigma.
Cell Culture.
Hamster insulinoma tumor HIT T15 cells were cultured at 37°C in RPMI medium 1640 containing 11 mM glucose and supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 2 mM glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin, 10−7 M selenous acid, and 10 μg/ml glutathione, as described (11). All experiments were carried out in HIT T15 cells of passage numbers between 73–80.
Cell Permeabilization and Measurements of Insulin Secretion.
Two different procedures were used to permeabilize cells, namely permeabilization by electric field and streptolysin O-induced permeabilization. The permeabilization buffer consisted of 140 mM potassium glutamate, 5 mM NaCl, 1.2 mM MgCl2, 10 mM EGTA, 25 mM Hepes, 0.025% albumin (pH 7.0). For streptolysin O-induced permeabilization, 1 mM dithiothreitol was added to the buffer. The electropermeabilization and permeabilization with streptolysin O were performed as described (12, 13).
Both types of permeabilization have their own benefits and drawbacks. Electropermeabilization creates only small holes in the plasma membrane, that prevents leakage of molecules >5 kDa from the cell. These holes reseal with time (14). By contrast, cells permeabilized with streptolysin O are leaking not only small molecules but also large proteins. Cells permeabilized by this procedure do not reseal (15). The use of one or the other of the procedures was dependent on the aim of each particular experiment.
Measurements of insulin secretion were performed in a permeabilization buffer containing additionally 2 mM MgATP and an ATP-regenerating system, 2 mM creatine phosphate, 10 unit/ml creatine phosphokinase, and Ca2+ concentrations in the range from 3 × 10−8 M to 1 × 10−5 M. Priming effects of InsP6 on insulin secretion were measured as follows. Cells were treated with different concentrations of InsP6 for 15 min in ATP-containing buffer and then insulin secretion was measured in ATP-free buffer without InsP6 at 30 nM or at 10 μM Ca2+ during 15 min. Insulin content was determined by radioimmunoassay using rat insulin as a standard. The level of insulin secretion at 30 nM Ca2+, ranging from 30–50 μunits/105 cells/15 min in different experiments, was taken as 100%.
Free Ca2+-concentration in solutions was measured with a Ca2+-selective electrode (model 93-20, Orion, Boston). As standard solutions, Ca2+-buffers (World Precision Instruments, Sarasota, FL) with known Ca2+ concentrations were used.
Measurements of Protein Kinase C (PKC) Activity.
PKC activity was assayed by measuring the transfer of 32P to a specific enzyme substrate. PKC Assay system (Promega) provided high specificity of the assay by using biotinylated peptide substrate and streptavidin-coated filters. Briefly, cells were washed several times in PBS buffer, resuspended in extraction buffer (25 mM Tris⋅HCl, pH 7.4/0.5 mM EDTA/0.5 mM EGTA/10 mM 2-mercaptoethanol/1 μg/ml leupeptin/1 μg/ml aprotinin/0.5 mM phenylmethylsulfonyl fluoride) and sonicated. Enzyme activity was assayed in the presence of phospholipids (0.35 mg/ml phosphatidylserine and 0.035 mg/ml diacylglycerol) and either without (2.5 mM EGTA and 0.1 mM EDTA) or with Ca2+ (4.5 mM CaCl2, 2.5 mM EGTA, and 0.1 mM EDTA) for 8 min at 30°C. Reactions were started by addition [γ-32P]ATP and terminated by spotting on streptavidin-coated filters. Incorporation of 32P into biotinylated substrate was assessed by liquid scintillation counting.
Presentation of Results.
Data analysis was performed using the program sigma plot 1.02 for Windows (Jandel, San Rafael, CA). All results are expressed as means ± SEM for the indicated number of observations. Statistical significance of differences between means were assessed by Student’s unpaired t test. Differences were considered significant at P < 0.05.
RESULTS
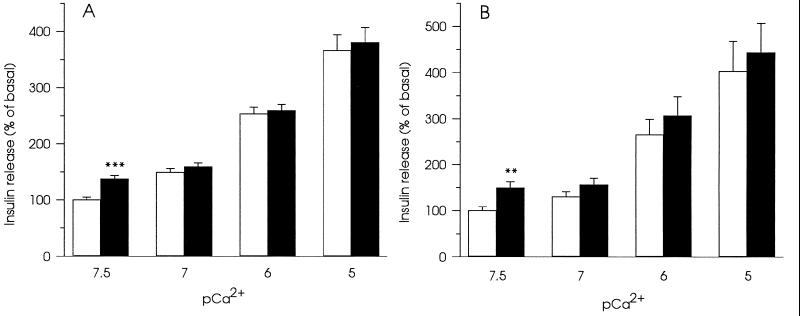
The effects of 20 μM InsP5 and 20 μM InsP6 on insulin secretion were tested at free Ca2+-concentrations ranging from 30 nM to 10 μM in electropermeabilized HIT T15 cells (Fig. 1). Only at basal (30 nM) free Ca2+ concentration, both compounds stimulated exocytosis of insulin. The stimulatory effect could not be detected at higher Ca2+-concentrations. Since InsP6 is present at high concentrations in insulin-secreting cells (4), this inositol polyphosphate was chosen for the investigation of the mechanism underlying the stimulatory effects of inositol polyphosphates on insulin secretion.
Figure 1.
Effects of 20 μM InsP5 (A) and 20 μM InsP6 (B) on insulin secretion in electropermeabilized HIT T15 cells at different free Ca2+-concentrations. Secretion without inositol polyphosphates is denoted by □ and with inositol polyphosphates by ▪. Data represent mean ± SEM for 16 observations from three separate experiments. ∗∗, P < 0.01 and ∗∗∗, P < 0.001, relative to insulin secretion without inositol polyphosphates.
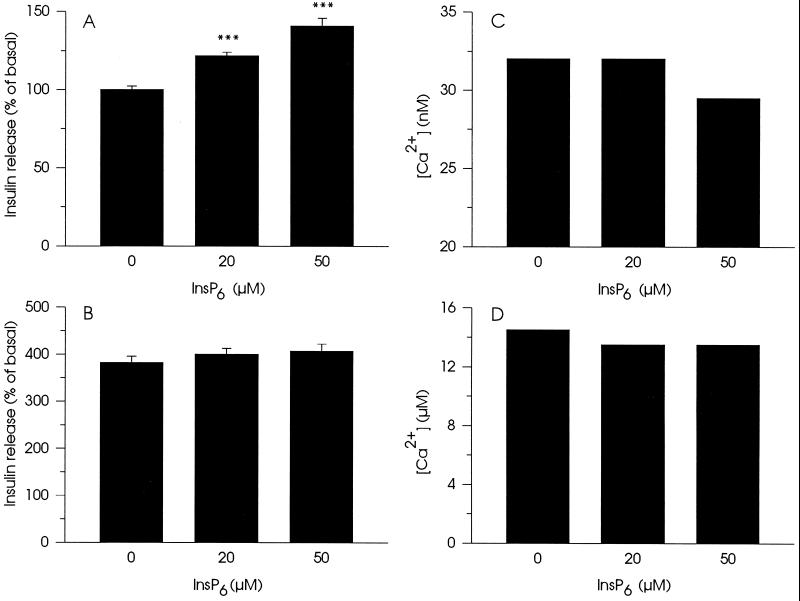
Stimulation of non-Ca2+-mediated insulin secretion by InsP6 was concentration-dependent and at 50 μM InsP6, insulin release reached ≈150% of secretion obtained in the absence of inositol polyphosphate (Fig. 2A). The stimulatory effects of InsP6 on insulin secretion could not be detected when the Ca2+-concentration was increased (Fig. 2B). To check if the observed effects of InsP6 were due to possible changes in free Ca2+-concentration induced by the compound, measurements of the ambient free Ca2+-concentration in the permeabilization buffer were performed (Fig. 2 C and D). At a concentration of 20 μM, InsP6 did not alter resting Ca2+-concentration (30 nM) (Fig. 2C). Under this condition 50 μM of InsP6 only reduced resting Ca2+-concentration by 20%. InsP6 (20 and 50 μM) decreased stimulating Ca2+-concentration not more than 7–10% (Fig. 2D). Although InsP6 has a well-documented ability to chelate Ca2+-ions (16), the Ca2+-buffers used in this study have enough capacity to prevent significant changes in free Ca2+-concentration in the presence of InsP6. Another Ca2+-chelator, 100 μM EGTA, added to the incubation buffer produced analogous slight decrease in free Ca2+-concentration but did not significantly affect insulin secretion measured at 30 nM Ca2+ (data not shown). Thus, the effects of InsP6 on insulin secretion cannot be accounted for by changes in the free Ca2+-concentration.
Figure 2.
Concentration-dependent effects of InsP6 on insulin secretion in electropermeabilized cells in the presence of either 30 nM (A) or 10 μM (B) Ca2+ and on free Ca2+-concentration in the permeabilization buffer containing either ≈30 nM (C) or ≈10 μM (D) Ca2+. For measurements of insulin secretion, data represent mean ± SEM for 18 observations from three separate experiments. ∗∗∗, P < 0.001, relative to insulin secretion without InsP6. For measurements of Ca2+, representative experiments of three are presented.
InsP6 is not the last member in the array of inositol polyphosphates. Higher phosphorylated inositol polyphosphate pyrophosphates, PP-InsP5 and (PP)2-InsP4, formed from InsP6 and InsP5, have also been described (17). It has been proposed that inositol polyphosphate pyrophosphates are intracellular energy stores because of the high energy hydrolysis of pyrophosphoryl residues (18). Therefore, we tested the specificity of the effect of InsP6 on exocytosis. The process of formation of pyrophosphates can be activated by NaF, through the inhibition of phosphatases responsible for pyrophosphate dephosphorylation (17). Addition of 5 mM NaF, this concentration maximally increased PP-InsP5 concentration but did not significantly activate G-proteins (17), instead of increasing slightly decreased InsP6-stimulated insulin release (139 ± 3% increase in secretion with 50 μM InsP6 vs. 127 ± 2% with 50 μM InsP6 and NaF, 15 observations from three separate experiments). This supports the suggestion that InsP6 rather than PP-InsP5 or (PP)2-InsP4 stimulated exocytosis.
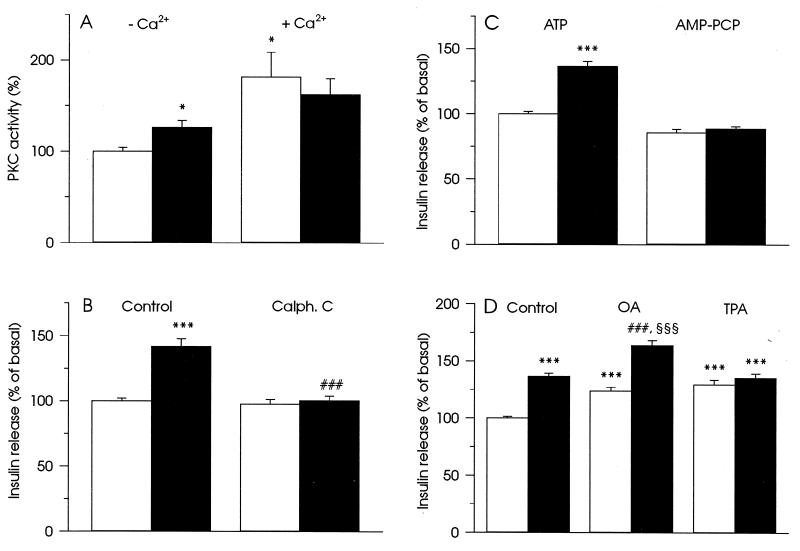
One of the reported effects of inositol polyphosphates, is activation of PKC isoenzymes as well as the PKC-related kinase, PRK1 (19). The kinase activation was observed only in a Ca2+-free medium and at inositol polyphosphate concentrations similar to those that stimulated insulin secretion in our experiments. Therefore, we examined the possibility that stimulation of exocytosis by inositol polyphosphates can be explained by activation of PKC. In HIT T15 cells, 50 μM InsP6 activated PKC in the absence of free Ca2+ and did not significantly affect Ca2+-induced activation of the enzyme (Fig. 3A).
Figure 3.
Involvement of PKC activation in the stimulation of insulin secretion in HIT T15 cells by InsP6. (A) Modulation of PKC activity by 50 μM InsP6 (▪) in the absence and the presence of free Ca2+ as described in Materials and Methods. (□) The enzyme activity without InsP6. Data represent mean ± SEM of four experiments. ∗, P < 0.05, relative to PKC activity in the absence of free Ca2+ and InsP6. (B) Inhibitor of PKC, 1.5 μM Calphostin C (Calph. C), blocked increase in insulin secretion induced by 50 μM InsP6. (C) Requirement of ATP-hydrolysis for the development of the stimulatory effect of 50 μM InsP6 on insulin secretion. ATP (2 mM) was substituted by 2 mM AMP-PCP, a nonhydrolyzable analog of ATP. (D) Additive effect of 50 μM InsP6 and 1 μM okadaic acid (OA) on stimulation of insulin secretion and the absence of additive effect of 50 μM InsP6 and 100 nM TPA. In B–D insulin secretion was measured in buffer with basal Ca2+ concentration (30 nM). (□) Insulin secretion without InsP6 and black columns illustrate insulin secretion with 50 μM InsP6. The presence of other compounds is indicated in the figure. For measurements of insulin secretion, data represent mean ± SEM for 17 (B), 18 (C), and 20 (D) observations from three separate experiments. ∗∗∗, P < 0.001, relative to insulin secretion at 30 nM Ca2+. ###, P < 0.001, relative to insulin secretion in the presence of 50 μM InsP6. §§§, P < 0.001, relative to insulin secretion in the presence of 1 μM OA.
The selective inhibitor of PKC, Calphostin C (1.5 μM) abolished stimulation of insulin secretion by 50 μM InsP6 (Fig. 3B). To confirm that protein kinase activation is involved in the stimulatory effect of InsP6 on insulin secretion, experiments substituting ATP with its non-hydrolyzable analog β,γ-methyleneadenosine 5′-triphosphate (AMP-PCP), which cannot serve as a substrate for protein kinase-induced phosphorylation, were performed. AMP-PCP blocked InsP6-induced exocytosis (Fig. 3C). Finally, 1 μM okadaic acid, an inhibitor of protein phosphatases type 1, 2A, and 3 (20), potentiated the effect of 50 μM InsP6 on insulin release, whereas 100 nM 12-O-tetradecanoylphorbol 13-acetate (TPA), a potent activator of PKC, did not (Fig. 3D). This suggests that activation of PKC rather than inhibition of protein phosphatases is responsible for the stimulation of exocytosis by InsP6.
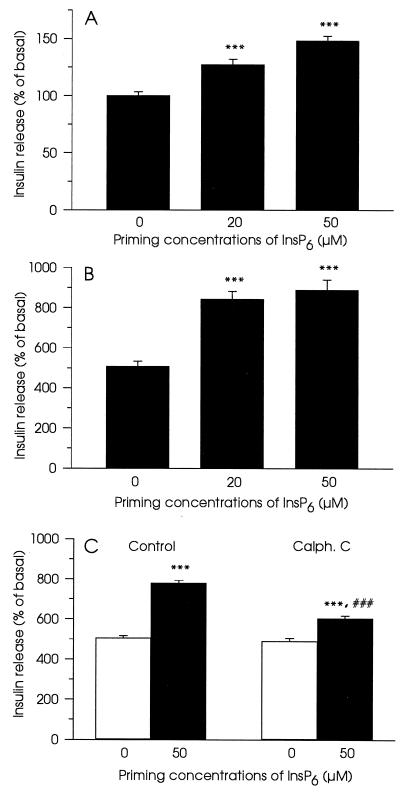
Experiments presented so far described acute effects of InsP6 on non-Ca2+-mediated insulin exocytosis. To evaluate possible effects of InsP6 on Ca2+-mediated exocytosis, cells were preincubated with the inositol polyphosphate in the absence of Ca2+ and subsequently stimulated with high concentrations of the ion. We have employed streptolysin O-permeabilized cells for this type of experiments, since the stability of the formed holes in the plasma membrane during prolonged time of incubation was essential. Permeabilized cells were first incubated in a buffer containing ATP, an ATP-regenerating system, 30 nM Ca2+, and different concentrations of InsP6. This buffer was replaced by a buffer containing 30 nM or 10 μM Ca2+ without InsP6 and ATP. Insulin content in the latter buffer was measured. Changing of permeabilization procedure did not perturb the ability of InsP6 to affect insulin exocytosis. InsP6 (20 and 50 μM) also primed non-Ca2+-mediated exocytosis (Fig. 4A). The effect of InsP6 on Ca2+-mediated exocytosis was pronounced. In this case, both 20 μM and 50 μM of the compound stimulated insulin secretion by 45% (Fig. 4B). Calphostin C (1.5 μM) suppressed insulin secretion induced by 50 μM InsP6 (Fig. 4C).
Figure 4.
Priming of insulin secretion by InsP6 in streptolysin O-permeabilized cells. Cells were pretreated with or without different concentrations of InsP6 for 15 min in ATP-containing buffer and then insulin secretion in ATP-free buffer was measured during 15 min at 30 nM (A) or at 10 μM Ca2+ in the absence of InsP6 (B and C). In C, 1.5 μM Calphostin C (Calph. C) was introduced together with InsP6. Data represent mean ± SEM for 15 observations from three separate experiments. ∗∗∗, P < 0.001, relative to insulin secretion without InsP6 and at appropriate Ca2+ concentration. ###, P < 0.001, relative to insulin secretion in the presence of InsP6.
DISCUSSION
Permeabilized insulin-secreting cells were used to investigate a possible role for InsP6 in regulated exocytosis. InsP6 stimulated non-Ca2+-mediated and primed Ca2+-mediated exocytosis of insulin, effects mediated through the activation of PKC. The involvement of PKC was verified by experiments showing that the InsP6 effect was blocked with Calphostin C and substitution of ATP with the non-hydrolyzable analog AMP-PCP. The potentiation of insulin secretion by InsP6 in the presence of 1 μM okadaic acid, a concentration known to inhibit protein phosphatases type 1, 2A, and 3 (20), suggests that the observed stimulatory activity of InsP6 on insulin release cannot be explained by an inhibitory activity of the compound on these protein phosphatases. Activation of PKC by InsP6 in insulin-secreting cells, in the absence of Ca2+, is consistent with the data that InsP6, at concentrations higher than 20 μM, activates several isoenzymes of PKC as well as PKC-related kinase (19).
Processes of protein phosphorylation play a central role in the regulation of exocytosis in the pancreatic β-cell (21, 22). Activation of PKC by InsP6 may lead to increased availability of releasable secretory granules, by promoting the recruitment and transport of granules to the site of exocytosis. The increasing amount of granules associated with the plasma membrane and ready-to-fuse would lead to the subsequent increase in insulin exocytosis. In addition, InsP6 may directly affect granule fusion since the conformation of proteins responsible for fusion events in exocytosis could be controlled by PKC activity (23, 24).
The fact that the stimulatory effect of InsP6 on insulin exocytosis disappeared at elevated Ca2+-concentrations, may be explained by the conversion of InsP6 into the inactive Ca2+-bound form, each molecule of InsP6 binding two or three Ca2+-ions (16). Such a conversion is in agreement with what has previously been suggested in the context of the disappearance of the stimulatory effect of inositol hexakisphosphate on PKC activity at high concentrations of Ca2+ (19). In the latter study, the authors suggested that the negative charge of phosphate groups plays a significant role in the effects of InsP6.
As discussed above, InsP6 readily chelates Ca2+-ions. This complicates any investigation of the role of the compound in Ca2+-mediated exocytosis. We have separated in time the preincubation of cells with InsP6 in ATP-containing medium and subsequent incubation of cells in the buffer containing high Ca2+-concentration in the absence of ATP and InsP6. The results obtained show that in HIT T15 cells Ca2+ triggers exocytosis of insulin in an ATP-independent manner, as described before for exocytosis of biogenic amines in PC12 cells (25–27). By applying this type of incubation procedure, we found that preincubation with InsP6 led to a markedly pronounced stimulatory effect on Ca2+-induced insulin exocytosis. It is likely that InsP6 under these conditions potentiated insulin release by priming Ca2+-mediated fusion of secretory granules with the plasma membrane. Hence, inositol polyphosphates may keep granule transport active under basal conditions and thereby increasing the number of granules available at the site of exocytosis, explaining both the stimulatory effect of InsP6 on non-Ca2+-mediated- and the priming of Ca2+-mediated insulin release (Fig. 5).
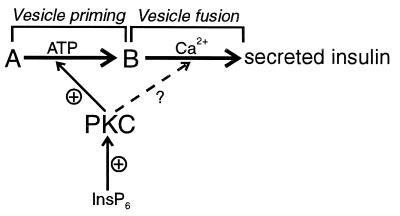
Figure 5.
Scheme illustrating the role of InsP6 in insulin exocytosis. A denotes an intracellular pool of insulin containing granules that are not activated for fusion, and B denotes a pool of granules close to the plasma membrane that undergoes Ca2+-activated fusion. The exocytotic process of insulin can thus be separated into ATP-dependent vesicle priming and ATP-independent Ca2+-activated fusion. According to the scheme the rate of insulin secretion is determined by the amount of primed vesicles and the probability of the fusion event for each granule. Through activation of PKC, InsP6 may influence both of these steps of insulin secretion. Hence, the processes discussed in this paper may be presented in the following way. (i) Insulin secretion at resting Ca2+ concentration and in the absence of InsP6. Insulin release is restricted by the amount of primed vesicles and a low probability of vesicle fusion at resting Ca2+ concentration. (ii) Activation of insulin secretion at resting Ca2+ concentration by InsP6. InsP6 activates PKC, which leads to an increased priming of secretory vesicles and maybe in addition direct activation of the fusion process. Modest stimulation of insulin release is observed relative to i. (iii) Ca2+-induced exocytosis in the absence of InsP6. Probability of vesicle fusion is high. It gives rise to a pronounced stimulation of insulin secretion, but the stimulation is still not maximal and limited by the availability of primed vesicles. (iv) Priming of Ca2+-induced exocytosis by InsP6. Similar to ii, InsP6 increased priming of secretory vesicles, an effect mediated by PKC activation. A high probability of vesicle fusion together with an increased amount of vesicles at the site of exocytosis lead to pronounced insulin secretion.
Intracellular levels of inositol polyphosphates vary, being particularly high in several cell types like the insulin-secreting cell. By influencing the rates of InsP6 synthesis and conversion, InsP6 concentration may be rapidly changed, which should in turn give rise to modulation of insulin exocytosis. We have shown that InsP6, in a physiological concentration range, is able to modulate exocytosis. At 20–50 μM concentrations, this compound stimulated insulin secretion at basal Ca2+-concentration and primed Ca2+-mediated exocytosis. Both effects of InsP6 were dependent on the activation of PKC. This suggests that inositol polyphosphates can function as signaling molecules in stimulus-secretion coupling in pancreatic β-cells.
Acknowledgments
We are grateful to Dr. C. J. Barker for fruitful discussions. This work was supported by the Swedish Medical Research Council (03X-09890 and 19X-00034), the Royal Swedish Academy of Sciences, the Swedish Foundation for International Cooperation in Research and Higher Education, the Swedish Diabetes Association, the Nordic Insulin Foundation Committee, Juvenile Diabetes Foundation International, and funds from the Karolinska Institute.
ABBREVIATIONS
- InsP5
d-myo-inositol 1,3,4,5,6-pentakisphosphate
- InsP6
d-myo-inositol 1,2,3,4,5,6-hexakisphosphate
- PKC
protein kinase C
- PP-InsP5 and (PP)2-InsP4
inositol polyphosphate pyrophosphates
- TPA
12-O-tetradecanoylphorbol 13-acetate
- AMP-PCP
β,γ-methyleneadenosine 5′-triphosphate
References
- 1.Sasakawa N, Sharif M, Hanley M R. Biochem Pharmacol. 1995;50:137–146. doi: 10.1016/0006-2952(95)00059-9. [DOI] [PubMed] [Google Scholar]
- 2.French P J, Bunce C M, Stephence L R, Lord J M, McConnell F M, Brown G, Creba J A, Michell R H. Proc R Soc London B. 1991;245:193–201. doi: 10.1098/rspb.1991.0109. [DOI] [PubMed] [Google Scholar]
- 3.Jackson T R, Hallam T J, Downes C P, Hanley M R. EMBO J. 1987;6:49–54. doi: 10.1002/j.1460-2075.1987.tb04717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G, Pralong W F, Pittet D, Mayr G W, Schlegel W, Wollheim C B. J Biol Chem. 1992;267:4349–4356. [PubMed] [Google Scholar]
- 5.Pittet D, Schlegel W, Lew D P, Monod A, Mayr G W. J Biol Chem. 1989;264:18489–18493. [PubMed] [Google Scholar]
- 6.Sasakawa N, Nakaki T, Kakinuma E, Kato R. Brain Res. 1993;623:155–160. doi: 10.1016/0006-8993(93)90023-g. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M, Aruga J, Niinobe M, Aimoto S, Mikoshiba K. J Biol Chem. 1994;269:29206–29211. [PubMed] [Google Scholar]
- 8.Fukuda M, Moreira J E, Lewis F M T, Sugimori M, Niinobe M, Mikoshiba K, Llinas R. Proc Natl Acad Sci USA. 1995;92:10708–10712. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck K A, Keen J H J. J Biol Chem. 1991;266:4442–4447. [PubMed] [Google Scholar]
- 10.Ye W, Ali N, Bembenek M E, Shears S B, Lafer E M. J Biol Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
- 11.Prentki M, Glennon M C, Geschwind J F, Matschinsky F M, Corkey B E. FEBS Lett. 1987;220:103–107. doi: 10.1016/0014-5793(87)80884-0. [DOI] [PubMed] [Google Scholar]
- 12.Vallar L, Biden T J, Wollheim C B. J Biol Chem. 1987;262:5049–5056. [PubMed] [Google Scholar]
- 13.Li G, Regazzi R, Balch W E, Wollheim C B. FEBS Lett. 1993;327:145–149. doi: 10.1016/0014-5793(93)80159-r. [DOI] [PubMed] [Google Scholar]
- 14.Knight D E, Scrutton M C. Biochem J. 1986;234:497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahnert-Hilger G, Stecher B, Beyer C, Gratzl M. Methods Enzymol. 1993;221:139–149. doi: 10.1016/0076-6879(93)21013-x. [DOI] [PubMed] [Google Scholar]
- 16.Luttrell B M. J Biol Chem. 1993;268:1521–1524. [PubMed] [Google Scholar]
- 17.Menniti F S, Miller R N, Putney J W, Shears S B. J Biol Chem. 1993;268:2850–3856. [PubMed] [Google Scholar]
- 18.Voglmaier S M, Bembenek M E, Kaplin A I, Dorman G, Olszewski J D, Prestwich G D, Snyder S H. Proc Natl Acad Sci USA. 1996;93:4305–4310. doi: 10.1073/pnas.93.9.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer R H, Dekker L V, Woscholski R, Le Good J A L, Gigg R, Parker P J. J Biol Chem. 1995;270:22412–22416. doi: 10.1074/jbc.270.38.22412. [DOI] [PubMed] [Google Scholar]
- 20.Bialojan C, Takai A. Biochem J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ämmälä C, Eliasson L, Bokvist K, Berggren P-O, Honkanen R E, Sjöholm Å, Rorsman P. Proc Natl Acad Sci USA. 1994;91:4343–4347. doi: 10.1073/pnas.91.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaitsev S V, Efendić S, Arkhammar P, Bertorello A M, Berggren P-O. Proc Natl Acad Sci USA. 1995;92:9712–9716. doi: 10.1073/pnas.92.21.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita Y, Sasaki T, Fukui K, Kotani H, Kimura T, Hata Y, Sudhof T C, Scheller R H, Takai Y. J Biol Chem. 1996;271:7265–7268. doi: 10.1074/jbc.271.13.7265. [DOI] [PubMed] [Google Scholar]
- 24.Shimazaki Y, Nishiki T, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- 25.Hay J C, Martin T F J. J Cell Biol. 1992;119:139–151. doi: 10.1083/jcb.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittner M A, Holz R W. J Biol Chem. 1992;267:16219–16225. [PubMed] [Google Scholar]
- 27.Thomas P, Wong J G, Lee A K, Almers W. Neuron. 1993;11:93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]