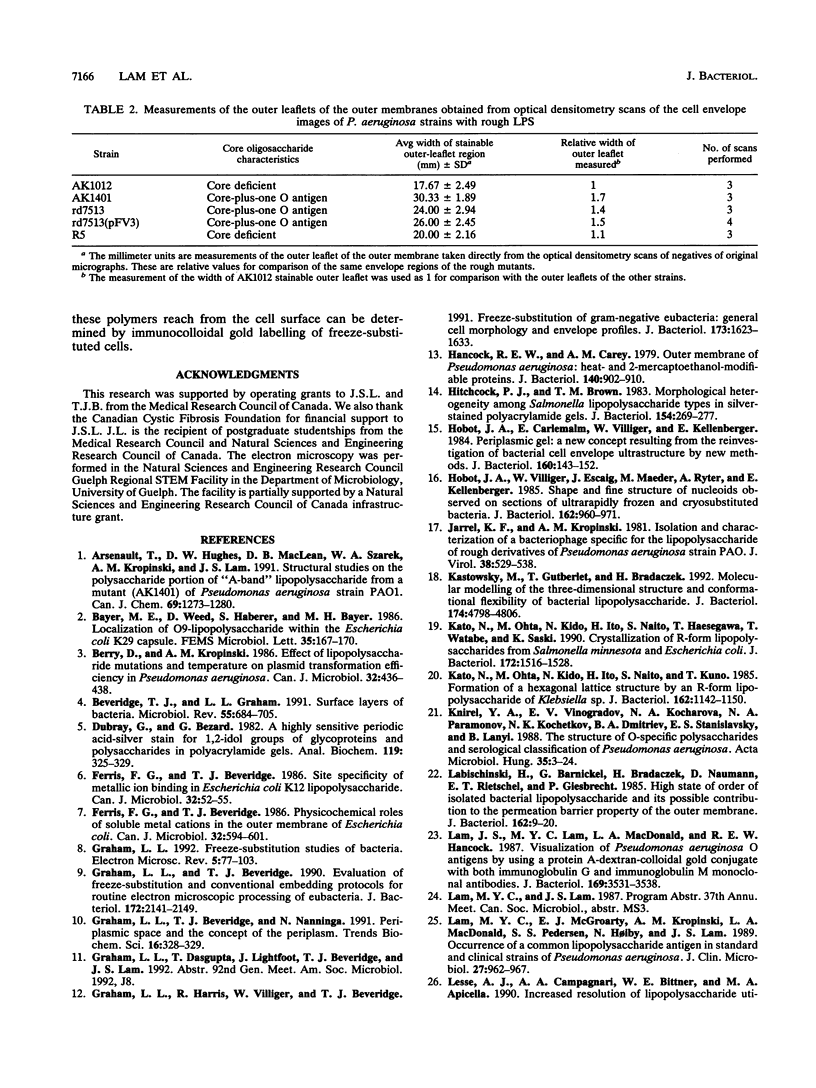
Abstract
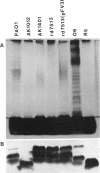
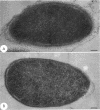
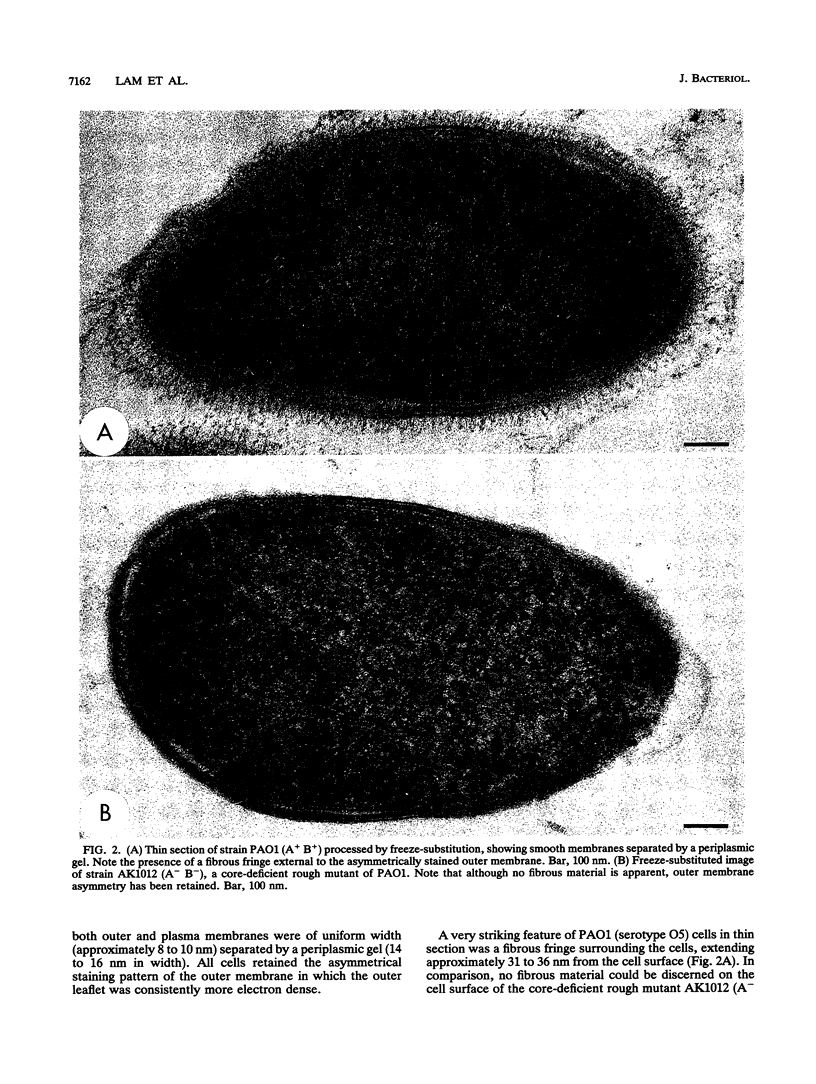
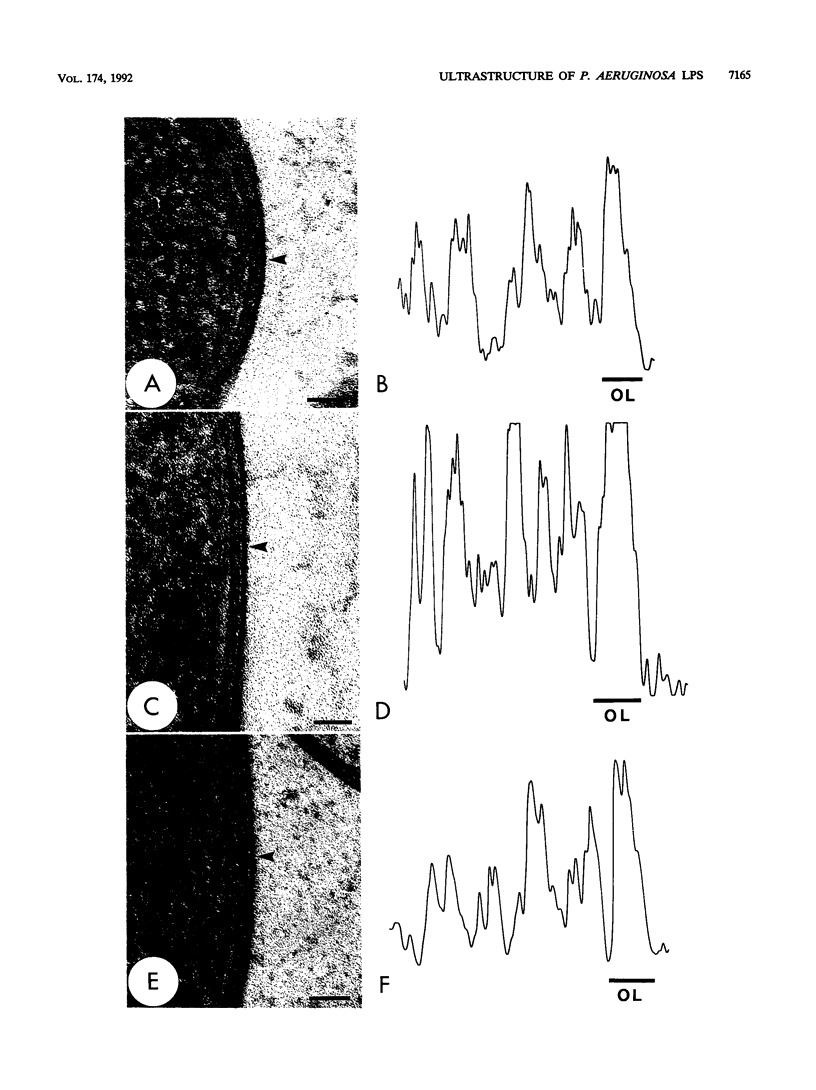
The majority of Pseudomonas aeruginosa strains synthesize two antigenically distinct types of lipopolysaccharide (LPS), namely, a serotype-specific B-band LPS and a common antigen A-band LPS. A-band LPS consists of uncharged poly-D-rhamnan, which does not bind uranyl ions and is difficult to stain for electron microscopy; the highly charged B-band LPS is more easily visualized. We selected two wild-type strains, PAO1 (serotype O5) and IATS O6 (serotype O6), generated isogenic mutants from them, and examined the distribution of LPS on the surface of these organisms by freeze-substitution and electron microscopy. On PAO1 cells, which express both A-band and B-band LPSs, a 31- to 36-nm-wide fringe extending perpendicularly from the outer membrane was observed. A fine fibrous material was also observed on the surface of serotype O6 (A+ B+) cells, although this material did not form a uniform layer. When the LPS-deficient mutants, strains AK1401 (A+ B-), AK 1012 (A- B-), rd7513 (A- B-), and R5 (an IATS O6-derived rough mutant; A- B-), were examined, no extraneous material was apparent above the bilayer. However, an asymmetrical staining pattern was observed on the outer leaflet of the outer membrane of each of these mutants, presumably conforming to the anionic charge distribution of the core region of the rough LPS. In all cases, expression of the LPS types was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining. When optical densitometry on electron microscopy negatives was used to analyze the outer membrane staining profiles, subtle differences in the degrees of core deficiency among rough mutants were detectable. This is the first time an electron microscopy technique has preserved the infrastructure produced in the outer membrane by its constituent macromolecules. We conclude that freeze-substitution electron microscopy is effective in the visualization of LPS morphotypes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry D., Kropinski A. M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986 May;32(5):436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Graham L. L. Surface layers of bacteria. Microbiol Rev. 1991 Dec;55(4):684–705. doi: 10.1128/mr.55.4.684-705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Ferris F. G., Beveridge T. J. Physicochemical roles of soluble metal cations in the outer membrane of Escherichia coli K-12. Can J Microbiol. 1986 Jul;32(7):594–601. doi: 10.1139/m86-110. [DOI] [PubMed] [Google Scholar]
- Ferris F. G., Beveridge T. J. Site specificity of metallic ion binding in Escherichia coli K-12 lipopolysaccharide. Can J Microbiol. 1986 Jan;32(1):52–55. doi: 10.1139/m86-010. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J., Nanninga N. Periplasmic space and the concept of the periplasm. Trends Biochem Sci. 1991 Sep;16(9):328–329. doi: 10.1016/0968-0004(91)90135-i. [DOI] [PubMed] [Google Scholar]
- Graham L. L. Freeze-substitution studies of bacteria. Electron Microsc Rev. 1992;5(1):77–103. doi: 10.1016/0892-0354(92)90006-c. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Harris R., Villiger W., Beveridge T. J. Freeze-substitution of gram-negative eubacteria: general cell morphology and envelope profiles. J Bacteriol. 1991 Mar;173(5):1623–1633. doi: 10.1128/jb.173.5.1623-1633.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Kropinski A. M. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J Virol. 1981 May;38(2):529–538. doi: 10.1128/jvi.38.2.529-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastowsky M., Gutberlet T., Bradaczek H. Molecular modelling of the three-dimensional structure and conformational flexibility of bacterial lipopolysaccharide. J Bacteriol. 1992 Jul;174(14):4798–4806. doi: 10.1128/jb.174.14.4798-4806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Ohta M., Kido N., Ito H., Naito S., Hasegawa T., Watabe T., Sasaki K. Crystallization of R-form lipopolysaccharides from Salmonella minnesota and Escherichia coli. J Bacteriol. 1990 Mar;172(3):1516–1528. doi: 10.1128/jb.172.3.1516-1528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Ohta M., Kido N., Ito H., Naito S., Kuno T. Formation of a hexagonal lattice structure by an R-form lipopolysaccharide of Klebsiella sp. J Bacteriol. 1985 Jun;162(3):1142–1150. doi: 10.1128/jb.162.3.1142-1150.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirel YuA, Vinogradov E. V., Kocharova N. A., Paramonov N. A., Kochetkov N. K., Dmitriev B. A., Stanislavsky E. S., Lányi B. The structure of O-specific polysaccharides and serological classification of Pseudomonas aeruginosa (a review). Acta Microbiol Hung. 1988;35(1):3–24. [PubMed] [Google Scholar]
- Labischinski H., Barnickel G., Bradaczek H., Naumann D., Rietschel E. T., Giesbrecht P. High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeation barrier property of the outer membrane. J Bacteriol. 1985 Apr;162(1):9–20. doi: 10.1128/jb.162.1.9-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., Lam M. Y., MacDonald L. A., Hancock R. E. Visualization of Pseudomonas aeruginosa O antigens by using a protein A-dextran-colloidal gold conjugate with both immunoglobulin G and immunoglobulin M monoclonal antibodies. J Bacteriol. 1987 Aug;169(8):3531–3538. doi: 10.1128/jb.169.8.3531-3538.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M. Y., McGroarty E. J., Kropinski A. M., MacDonald L. A., Pedersen S. S., Høiby N., Lam J. S. Occurrence of a common lipopolysaccharide antigen in standard and clinical strains of Pseudomonas aeruginosa. J Clin Microbiol. 1989 May;27(5):962–967. doi: 10.1128/jcm.27.5.962-967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot J., Lam J. S. Molecular cloning of genes involved with expression of A-band lipopolysaccharide, an antigenically conserved form, in Pseudomonas aeruginosa. J Bacteriol. 1991 Sep;173(18):5624–5630. doi: 10.1128/jb.173.18.5624-5630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., McGroarty E. J. Analysis of a common-antigen lipopolysaccharide from Pseudomonas aeruginosa. J Bacteriol. 1989 Apr;171(4):2244–2248. doi: 10.1128/jb.171.4.2244-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Graham J. A., Nath K. The morphologic structure of isolated bacterial lipopolysaccharide. J Mol Biol. 1967 Apr 14;25(1):15–21. doi: 10.1016/0022-2836(67)90275-6. [DOI] [PubMed] [Google Scholar]
- Shands J. W. Localization of Somatic Antigen on Gram-Negative Bacteria by Electron Microscopy. J Bacteriol. 1965 Jul;90(1):266–270. doi: 10.1128/jb.90.1.266-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C., Schoenhals G., Graham L. Mutants of Escherichia coli O9:K30 with altered synthesis and expression of the capsular K30 antigen. J Gen Microbiol. 1989 Oct;135(10):2589–2599. doi: 10.1099/00221287-135-10-2589. [DOI] [PubMed] [Google Scholar]