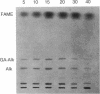
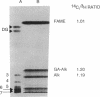
Abstract
Deinococcus radiodurans contains novel phospholipids of which the structures of three have been previously described. These three lipids contain both fatty acids and alkylamines. Both the fatty acid and alkylamine constituents were found to be composed of a mixture of species, of which C15, C16, and C17 saturated and monounsaturated alkyl chains predominated. Alkylamines contained a relatively higher proportion of saturated species. Progression of bacterial growth through the mid-log to stationary phases was accompanied by an increase in the proportions of C15 and C17 alkyl chains in both fatty acid and alkylamine constituents. Radiolabeled palmitic acid was found to be rapidly incorporated into both fatty acid and alkylamine components of phosphatidylglyceroylalkylamine, which is the precursor of the more-complex phosphoglycolipids found in major amounts in D. radiodurans. After culturing D. radiodurans in the presence of a mixture of palmitic acids labeled with 14C and 3H in the 1 and 9,10 positions, respectively, the same 14C/3H ratio was recovered in both fatty acid and alkylamine constituents, strongly suggesting that alkylamines are derived from intact fatty acids rather than by a de novo pathway. The results identify a novel product of fatty acid metabolism which has not to date been observed in any other organism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Hansen K. Structure of a novel phosphoglycolipid from Deinococcus radiodurans. J Biol Chem. 1985 Oct 5;260(22):12219–12223. [PubMed] [Google Scholar]
- Anderson R., Kates M., Volcani B. E. Identification of the sulfolipids in the non-photosynthetic diatom Nitzschia alba. Biochim Biophys Acta. 1978 Jan 27;528(1):89–106. doi: 10.1016/0005-2760(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Anderson R., Livermore B. P., Volcani B. E., Kates M. A novel sulfonolipid in diatoms. Biochim Biophys Acta. 1975 Nov 21;409(2):259–263. doi: 10.1016/0005-2760(75)90160-5. [DOI] [PubMed] [Google Scholar]
- Andersson B. A., Holman R. T. Pyrrolidides for mass spectrometric determination of the position of the double bond in monounsaturated fatty acids. Lipids. 1974 Mar;9(3):185–190. doi: 10.1007/BF02532690. [DOI] [PubMed] [Google Scholar]
- Christensen E. A., Kristensen H. Radiation-resistance of micro-organisms from air in clean premises. Acta Pathol Microbiol Scand B. 1981 Oct;89(5):293–301. doi: 10.1111/j.1699-0463.1981.tb00192_89b.x. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Phospholipid alterations during growth of Escherichia coli. J Bacteriol. 1968 Jun;95(6):2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowfoot P. D., Hunt A. L. The effect of oxygen tension on methylene hexadecanoic acid formation in Pseudomonas fluorescens and Escherichia coli. Biochim Biophys Acta. 1970 May 5;202(3):550–552. doi: 10.1016/0005-2760(70)90127-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fulco A. J. Fatty acid metabolism in bacteria. Prog Lipid Res. 1983;22(2):133–160. doi: 10.1016/0163-7827(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J., Gill D. L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990 Jun 29;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Leadbetter E. R. Sulfonolipids of gliding bacteria. Structure of the N-acylaminosulfonates. J Biol Chem. 1984 Mar 10;259(5):2982–2990. [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Huang Y., Anderson R. Phosphatidylglyceroylalkylamine, a novel phosphoglycolipid precursor in Deinococcus radiodurans. J Bacteriol. 1991 Jan;173(2):457–462. doi: 10.1128/jb.173.2.457-462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Anderson R. Structure of a novel glucosamine-containing phosphoglycolipid from Deinococcus radiodurans. J Biol Chem. 1989 Nov 5;264(31):18667–18672. [PubMed] [Google Scholar]
- Ingram L. O., Chevalier L. S., Gabba E. J., Ley K. D., Winters K. Propionate-induced synthesis of odd-chain-length fatty acids by Escherichia coli. J Bacteriol. 1977 Sep;131(3):1023–1025. doi: 10.1128/jb.131.3.1023-1025.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungkind D. L., Wood R. C. Factors involved in the synthesis of cyclopropane fatty acids by Streptococcus faecalis. Biochim Biophys Acta. 1974 Feb 25;337(2):286–297. doi: 10.1016/0005-2760(74)90210-0. [DOI] [PubMed] [Google Scholar]
- KATES M., ADAMS G. A., MARTIN S. M. LIPIDS OF SERRATIA MARCESCENS. Can J Biochem. 1964 Apr;42:461–479. doi: 10.1139/o64-054. [DOI] [PubMed] [Google Scholar]
- Knivett V. A., Cullen J. Fatty acid synthesis in Escherichia coli. Biochem J. 1967 May;103(2):299–306. doi: 10.1042/bj1030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen H., Christensen E. A. Radiation-resistant micro-organisms isolated from textiles. Acta Pathol Microbiol Scand B. 1981 Oct;89(5):303–309. doi: 10.1111/j.1699-0463.1981.tb00193_89b.x. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. I., Murray R. G., Moseley B. E., Minton K. W. DNA polymorphisms in new isolates of 'Deinococcus radiopugnans'. J Gen Microbiol. 1991 Jul;137(7):1459–1469. doi: 10.1099/00221287-137-7-1459. [DOI] [PubMed] [Google Scholar]
- Pinto W. J., Srinivasan B., Shepherd S., Schmidt A., Dickson R. C., Lester R. L. Sphingolipid long-chain-base auxotrophs of Saccharomyces cerevisiae: genetics, physiology, and a method for their selection. J Bacteriol. 1992 Apr;174(8):2565–2574. doi: 10.1128/jb.174.8.2565-2574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., LeKim D., Sticht G. Biosynthesis of dihydrosphingosine in vitro. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):664–670. doi: 10.1515/bchm2.1968.349.1.664. [DOI] [PubMed] [Google Scholar]
- Stumpf P. K. Metabolism of fatty acids. Annu Rev Biochem. 1969;38:159–212. doi: 10.1146/annurev.bi.38.070169.001111. [DOI] [PubMed] [Google Scholar]
- Thompson B. G., Anderson R., Murray R. G. Unusual polar lipids of Micrococcus radiodurans strain Sark. Can J Microbiol. 1980 Dec;26(12):1408–1411. doi: 10.1139/m80-234. [DOI] [PubMed] [Google Scholar]