Abstract
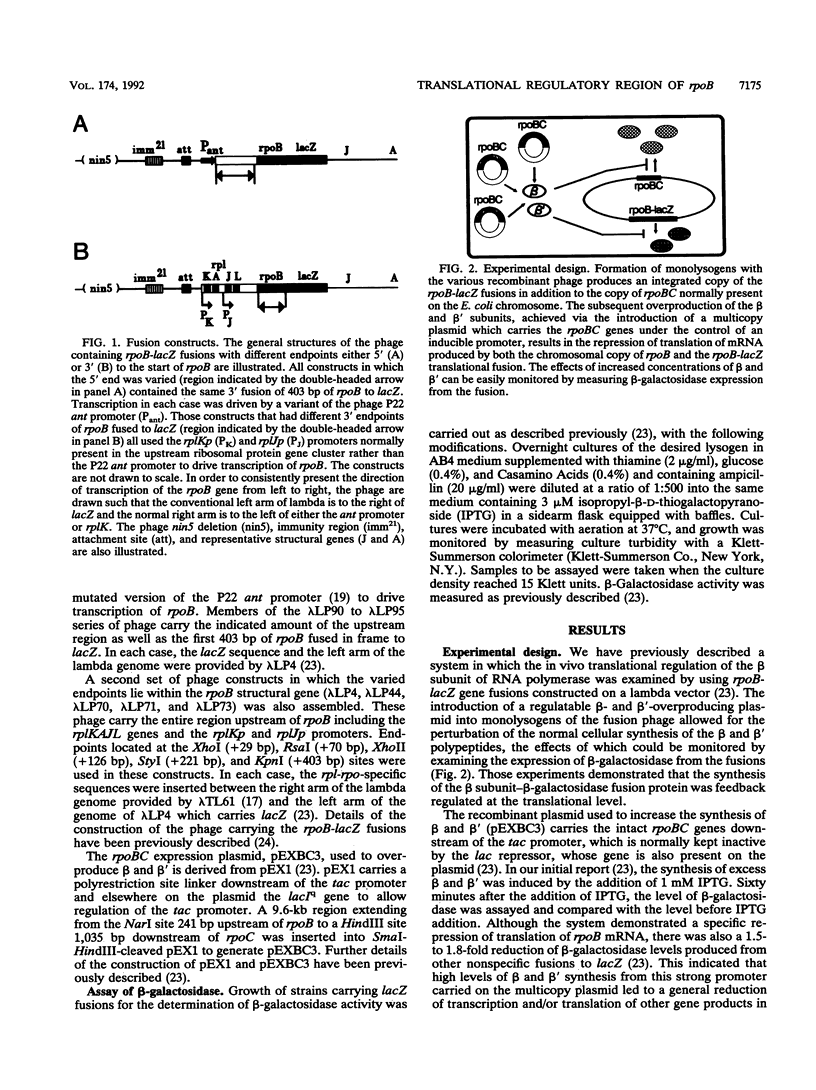
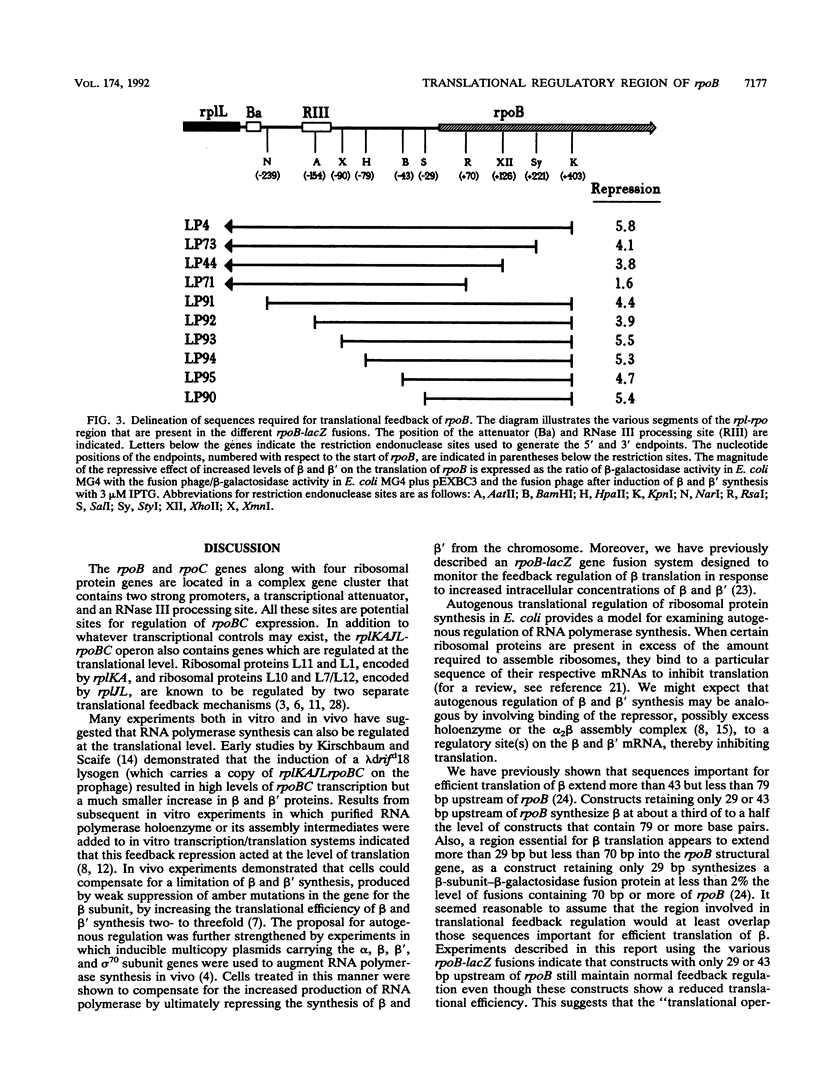
In order to delineate the region involved in feedback regulation of the RNA polymerase beta subunit (encoded by rpoB), a collection of rpoB-lacZ translational fusions with different endpoints both upstream and downstream of the rpoB start site was assembled on lambda phage vectors. The extent of translational repression of beta was monitored by measuring beta-galactosidase levels in monolysogens of the fusions under conditions of increased intracellular concentrations of beta and beta' achieved via the induction of rpoBC expression from a multicopy plasmid. A construct containing as little as 29 bp upstream of the start of rpoB exhibited repression of beta-galactosidase activity to the same extent as a construct encoding the full upstream region. A construct which carried only 70 bp of the rpoB structural gene exhibited very little repression, while constructs which carried 126 or 221 bp of rpoB exhibited approximately the same degree of repression as a construct which carried 403 bp. These data suggest that the sequences important for feedback regulation of beta translation extend more than 70 bp into rpoB but are completely contained within a region which spans the sequences from 29 bp upstream to 126 bp downstream of the translational start site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry G., Squires C. L., Squires C. Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4922–4926. doi: 10.1073/pnas.76.10.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C., Squires C. L. Attenuation and processing of RNA from the rplJL--rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3331–3335. doi: 10.1073/pnas.77.6.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Bedwell D. M., Nomura M. Feedback regulation of RNA polymerase subunit synthesis after the conditional overproduction of RNA polymerase in Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):17–23. doi: 10.1007/BF00330181. [DOI] [PubMed] [Google Scholar]
- Carter-Muenchau P., Wolf R. E., Jr Growth-rate-dependent regulation of 6-phosphogluconate dehydrogenase level mediated by an anti-Shine-Dalgarno sequence located within the Escherichia coli gnd structural gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1138–1142. doi: 10.1073/pnas.86.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D., Nomura M. Feedback regulation of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3590–3594. doi: 10.1073/pnas.77.6.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nene V., Glass R. E. Autogenous posttranscriptional regulation of RNA polymerase beta and beta' subunit synthesis in Escherichia coli. J Bacteriol. 1985 Feb;161(2):803–806. doi: 10.1128/jb.161.2.803-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R., Taketo M., Ishihama A. Autogenous regulation of RNA polymerase beta subunit synthesis in vitro. J Biol Chem. 1978 Jul 10;253(13):4501–4504. [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Straus D. B., Walter W. A., Gross C. A. Sigma 32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987 Apr;1(2):179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- Johnsen M., Christensen T., Dennis P. P., Fiil N. P. Autogenous control: ribosomal protein L10-L12 complex binds to the leader sequence of its mRNA. EMBO J. 1982;1(8):999–1004. doi: 10.1002/j.1460-2075.1982.tb01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Fukuda R., Ishihama A. Autogenous and post-transcriptional regulation of Escherichia coli RNA polymerase synthesis in vitro. Mol Gen Genet. 1980;179(3):489–496. doi: 10.1007/BF00271738. [DOI] [PubMed] [Google Scholar]
- Kamath-Loeb A. S., Gross C. A. Translational regulation of sigma 32 synthesis: requirement for an internal control element. J Bacteriol. 1991 Jun;173(12):3904–3906. doi: 10.1128/jb.173.12.3904-3906.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Scaife J. Evidence for a lambda transducing phage carrying the genes for the beta and beta' subunits of Escherichia coli RNA polymerase. Mol Gen Genet. 1974;132(3):193–201. doi: 10.1007/BF00269392. [DOI] [PubMed] [Google Scholar]
- Lang-Yang H., Zubay G. Negative regulation of beta and beta' synthesis by RNA polymerase. Mol Gen Genet. 1981;183(3):514–517. doi: 10.1007/BF00268773. [DOI] [PubMed] [Google Scholar]
- Linn T., St Pierre R. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J Bacteriol. 1990 Feb;172(2):1077–1084. doi: 10.1128/jb.172.2.1077-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek D. W., Hayward R. S. Direct evidence for autogenous regulation of the Escherichia coli genes rpoBC in vivo. Mol Gen Genet. 1986 Mar;202(3):500–508. doi: 10.1007/BF00333284. [DOI] [PubMed] [Google Scholar]
- Moyle H., Waldburger C., Susskind M. M. Hierarchies of base pair preferences in the P22 ant promoter. J Bacteriol. 1991 Mar;173(6):1944–1950. doi: 10.1128/jb.173.6.1944-1950.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H., Yuzawa H., Yura T. Interplay of two cis-acting mRNA regions in translational control of sigma 32 synthesis during the heat shock response of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10515–10519. doi: 10.1073/pnas.88.23.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Otsuka J., Suzuki H., Kunisawa T. Regulation in the synthesis of Escherichia coli RNA polymerase proposed from sequence analysis. Protein Seq Data Anal. 1988;1(5):355–361. [PubMed] [Google Scholar]
- Passador L., Linn T. Autogenous regulation of the RNA polymerase beta subunit of Escherichia coli occurs at the translational level in vivo. J Bacteriol. 1989 Nov;171(11):6234–6242. doi: 10.1128/jb.171.11.6234-6242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralling G., Bodrug S., Linn T. Growth rate-dependent regulation of RNA polymerase synthesis in Escherichia coli. Mol Gen Genet. 1985;201(3):379–386. doi: 10.1007/BF00331327. [DOI] [PubMed] [Google Scholar]
- Steward K. L., Linn T. In vivo analysis of overlapping transcription units in the rplKAJLrpoBC ribosomal protein-RNA polymerase gene cluster of Escherichia coli. J Mol Biol. 1991 Mar 5;218(1):23–31. doi: 10.1016/0022-2836(91)90870-c. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. The heat shock response of E. coli is regulated by changes in the concentration of sigma 32. Nature. 1987 Sep 24;329(6137):348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]


