Abstract
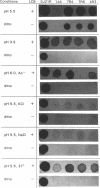
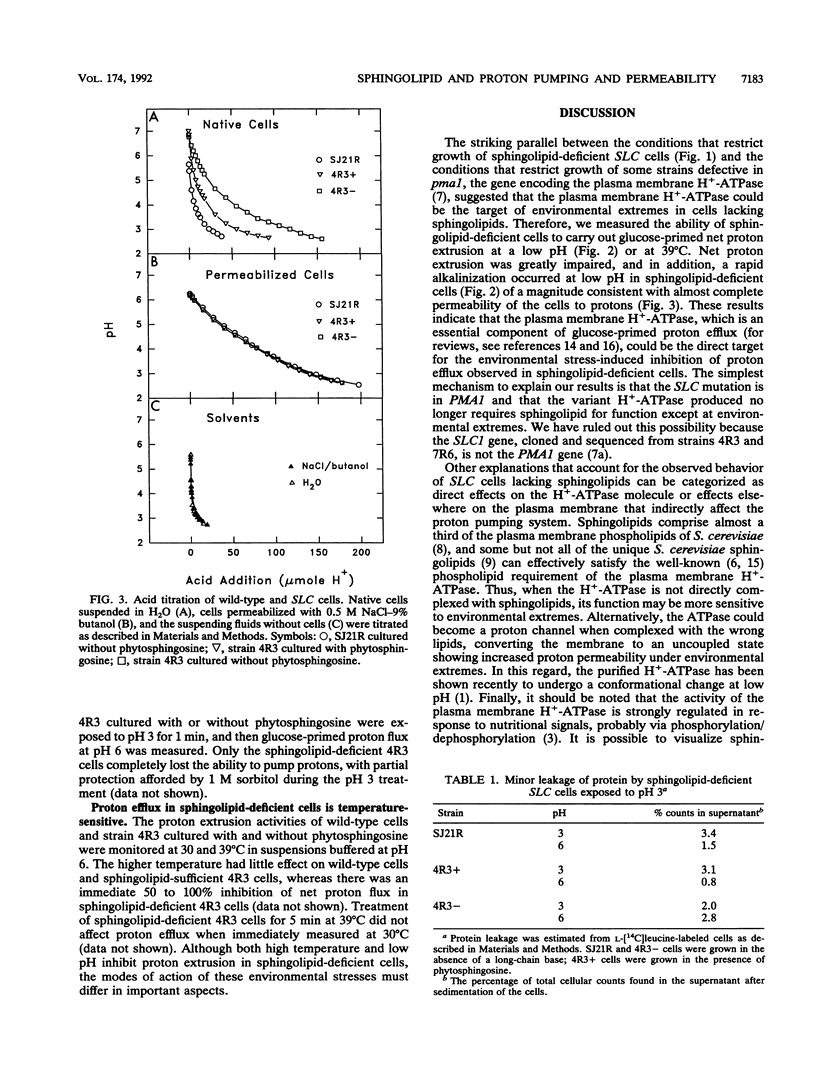
To study sphingolipid function(s) in Saccharomyces cerevisiae, we have investigated the effects of environmental stress on mutant (SLC) strains (R. C. Dickson, G. B. Wells, A. Schmidt, and R. L. Lester, Mol. Cell. Biol. 10:2176-2181, 1990) that either contain or lack sphingolipids, depending on whether they are cultured with a sphingolipid long-chain base. Strains lacking sphingolipid were unable to grow at low pH, at 37 degrees C, or with high salt concentrations in the medium; these environmental stresses are known to inhibit the growth of some S. cerevisiae strains with a defective plasma membrane H(+)-ATPase. We found that sphingolipids were essential for proton extrusion at low pH and furthermore found that cells lacking sphingolipid no longer exhibited net proton extrusion at normal pH after a 1-min exposure to pH 3. Cells lacking sphingolipid appeared to rapidly become almost completely permeable to protons at low pH. The deleterious effects of low pH could be partially prevented by 1 M sorbitol in the suspension of cells lacking sphingolipid. Proton extrusion at normal pH (pH 6) was significantly inhibited at 39 degrees C only in cells lacking sphingolipid. Thus, the product of an SLC suppressor gene permits life without sphingolipids only in a limited range of environments. Outside this range, sphingolipids appear to be essential for maintaining proton permeability barriers and/or for proton extrusion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanpain J. P., Ronjat M., Supply P., Dufour J. P., Goffeau A., Dupont Y. The yeast plasma membrane H(+)-ATPase. An essential change of conformation triggered by H+. J Biol Chem. 1992 Feb 25;267(6):3735–3740. [PubMed] [Google Scholar]
- Buede R., Rinker-Schaffer C., Pinto W. J., Lester R. L., Dickson R. C. Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. J Bacteriol. 1991 Jul;173(14):4325–4332. doi: 10.1128/jb.173.14.4325-4332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Slayman C. W. Maturation of the yeast plasma membrane [H+]ATPase involves phosphorylation during intracellular transport. J Cell Biol. 1991 Oct;115(2):289–295. doi: 10.1083/jcb.115.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid A., Serrano R. Mutations of the yeast plasma membrane H+-ATPase which cause thermosensitivity and altered regulation of the enzyme. J Biol Chem. 1988 Oct 5;263(28):14134–14139. [PubMed] [Google Scholar]
- Dickson R. C., Wells G. B., Schmidt A., Lester R. L. Isolation of mutant Saccharomyces cerevisiae strains that survive without sphingolipids. Mol Cell Biol. 1990 May;10(5):2176–2181. doi: 10.1128/mcb.10.5.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpartida F., Serrano R. Purification of the yeast plasma membrane ATPase solubilized with a novel zwitterionic detergent. FEBS Lett. 1980 Feb 25;111(1):69–72. doi: 10.1016/0014-5793(80)80763-0. [DOI] [PubMed] [Google Scholar]
- McCusker J. H., Perlin D. S., Haber J. E. Pleiotropic plasma membrane ATPase mutations of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Nov;7(11):4082–4088. doi: 10.1128/mcb.7.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. L., Lester R. L. Phosphatidylinositol phosphate, phosphatidylinositol bisphosphate, and the phosphoinositol sphingolipids are found in the plasma membrane and stimulate the plasma membrane H(+)-ATPase of Saccharomyces cerevisiae. Arch Biochem Biophys. 1992 Jan;292(1):70–76. doi: 10.1016/0003-9861(92)90052-x. [DOI] [PubMed] [Google Scholar]
- Patton J. L., Lester R. L. The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J Bacteriol. 1991 May;173(10):3101–3108. doi: 10.1128/jb.173.10.3101-3108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto W. J., Srinivasan B., Shepherd S., Schmidt A., Dickson R. C., Lester R. L. Sphingolipid long-chain-base auxotrophs of Saccharomyces cerevisiae: genetics, physiology, and a method for their selection. J Bacteriol. 1992 Apr;174(8):2565–2574. doi: 10.1128/jb.174.8.2565-2574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto W. J., Wells G. W., Lester R. L. Characterization of enzymatic synthesis of sphingolipid long-chain bases in Saccharomyces cerevisiae: mutant strains exhibiting long-chain-base auxotrophy are deficient in serine palmitoyltransferase activity. J Bacteriol. 1992 Apr;174(8):2575–2581. doi: 10.1128/jb.174.8.2575-2581.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Sancho E. D. Cation exchanges of yeast in the absence of magnesium. Biochim Biophys Acta. 1979 Apr 4;552(2):322–330. doi: 10.1016/0005-2736(79)90286-4. [DOI] [PubMed] [Google Scholar]
- Serrano R. Effect of ATPase inhibitors on the proton pump of respiratory-deficient yeast. Eur J Biochem. 1980 Apr;105(2):419–424. doi: 10.1111/j.1432-1033.1980.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Sigler K., Höfer M. Mechanisms of acid extrusion in yeast. Biochim Biophys Acta. 1991 Dec 12;1071(4):375–391. doi: 10.1016/0304-4157(91)90003-f. [DOI] [PubMed] [Google Scholar]
- Smith S. W., Lester R. L. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J Biol Chem. 1974 Jun 10;249(11):3395–3405. [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S., Smith S., Waechter C. J., Lester R. L. Isolation and partial characterization of a major inositol-containing lipid in baker's yeast, mannosyl-diinositol, diphosphoryl-ceramide. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1042–1048. doi: 10.1073/pnas.64.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. B., Lester R. L. The isolation and characterization of a mutant strain of Saccharomyces cerevisiae that requires a long chain base for growth and for synthesis of phosphosphingolipids. J Biol Chem. 1983 Sep 10;258(17):10200–10203. [PubMed] [Google Scholar]