Abstract
Hepatic metabolism and gene expression are among other regulatory mechanisms controlled by the cellular hydration state, which changes rapidly in response to anisotonicity, concentrative substrate uptake, oxidative stress, and under the influence of hormones such as insulin and glucagon. Differential screening for cell volume sensitive transcripts in a human hepatoma cell line revealed a gene for a putative serine/threonine kinase, h-sgk, which has 98% sequence identity to a serum- and glucocorticoid regulated kinase, sgk, cloned from a rat mammary tumor cell line. h-sgk transcript levels were strongly altered during anisotonic and isotonic cell volume changes. Within 30 min h-sgk RNA was, independent of de novo protein synthesis, induced upon cell shrinkage and, due to a complete stop in h-sgk transcription, reduced upon cell swelling. Comparable changes of sgk transcript levels were observed in a renal epithelial cell line. h-sgk mRNA was detected in all human tissues tested, with the highest levels in pancreas, liver, and heart. The putative serine/threonine protein kinase h-sgk may provide a functional link between the cellular hydration state and metabolic control.
Keywords: signal transduction, RNA fingerprinting, transcriptional regulation, hepatocytes/MDCK cells
Even at constant extracellular osmolarity, cell volume constancy is challenged by transport across the cell membrane and cellular metabolism leading to formation or disposal of osmotically active substances (1). Cell swelling or shrinkage disturb the intracellular milieu by dilution and concentration, respectively, of cellular macromolecules leading to profound alterations of cellular function (2–6). Thus, cells have developed a variety of cell volume regulatory mechanisms. In most tissues, cell swelling leads to a release of ions through activation of ion channels and/or KCl cotransport and cell shrinkage stimulates ion uptake through activation of cation channels, NaCl/KCl cotransport and Na+/H+ exchange (1). Moreover, cell shrinkage stimulates the accumulation and cell swelling the release or disposal of osmolytes, molecules specifically designed to create intracellular osmolarity (7). Beyond that, alterations of hepatocyte cell volume profoundly modulate hepatocellular metabolism and gene expression (8, 9). Cell swelling acts like an anabolic signal, stimulating protein and glycogen synthesis and simultaneously inhibiting glycogenolysis and proteolysis (8, 10). Cell shrinkage, on the other hand, is a catabolic stimulus, activating glycogenolysis and proteolysis and inhibiting protein and glycogen synthesis (8). Genes preferably expressed in shrunken cells include the phosphoenolpyruvate carboxykinase (11), genes preferably expressed in swollen cells include ornithine decarboxylase (12) and the transcription factor c-jun (13). Cell volume has been recognized as a decisive element in the regulation of hepatocellular metabolism by hormones (8, 14), cumulative amino acid uptake (15), and oxidative stress (16, 17).
The signaling mechanisms that link cell function to changes of liver cell hydration are still ill defined. Changes in cell volume may exert some of their pleiotropic actions through the induction or repression of regulatory genes, whose products could in turn influence the expression or activity of a wide variety of cellular components. To test this hypothesis, a differential RNA fingerprinting assay was performed on cDNAs isolated from hepatocytes exposed to isotonic and anisotonic media to identify and characterize genes that are transcriptionally regulated by the cellular hydration state.
MATERIALS AND METHODS
Materials.
Fetal bovine serum and DMEM were obtained from GIBCO/BRL. Enzymes were purchased from Stratagene and Boehringer Mannheim, α-[35S]dATP was obtained from ICN, and SuperScript reverse transcriptase was purchased from GIBCO/BRL. PCR reactions were performed in a Crocodile II thermocycler (Appligene Oncor) using Prime Zyme DNA polymerase and PCR buffer from Biometra (Göttingen, Germany). RNA arbitrarily primed (RAP)–PCR primers were purchased from Stratagene, sequencing primers from MWG Biotec (Ebersberg, Germany). Manual sequencing was performed on an S2 sequencing apparatus from GIBCO/BRL using the Fidelity DNA sequencing system (Appligene Oncor).
Cell Culture.
HepG2 human hepatoma cells were maintained in DMEM/5% CO2/5 mM glucose at 37°C, pH 7.4, supplemented with 10% (vol/vol) fetal calf serum (FCS). Prior to RNA isolation, the cells were grown to 90% confluence and shifted into basal medium Eagle (BME, GIBCO/BRL) without FCS for 12 hr. Hypertonic and hypotonic treatment was achieved by addition or removal of defined amounts of NaCl without changing the other components of BME. In experiments where the effects of amino acid addition were tested, the cells were kept in an amino acid free BME formulation 2 hr prior to amino acid addition.
RAP–PCR.
RNA fingerprinting by arbitrarily primed PCR (RAP–PCR) was performed as described (18). After electrophoresis through a 4% acrylamide/7 M urea denaturing polyacrylamide gel, the PCR products were visualized with a modified silver staining procedure (19). Any band clearly evident under one condition and absent in the other was subsequently confirmed by repeated reverse transcription and PCR using RNA isolated from new cultures. The RAP–PCR procedure was performed with four different primer pairs for cDNA synthesis and PCR amplification. Additionally, different annealing temperatures for the first round of amplification were used, ranging between 30°C and 40°C. Together with these modifications, a total of 64 PCRs were performed.
Band Recovery.
Bands demonstrating reproducible differences were excised under sterile conditions. The amplicon was eluted overnight at 70°C in 100 μl of elution buffer (50 mM KCl/10 mM TRIS⋅Cl, pH 9.0/0.1% Triton X-100). Reamplification by PCR was performed using 3.0 μl of eluate, the appropriate primer (250 nM), 200 μM dNTP, 1× low salt buffer (Stratagene) with 1.5 mM MgCl2 and 5 units of Taq+ DNA polymerase (Stratagene) with the following temperature cycling profile: 1 cycle of 95°C for 60 sec, 30 cycles of 95°C (15 sec), 55°C (15 sec), 72°C (60 sec), and the last extension at 72°C for 5 min. After confirmation by PAGE that only one defined amplicon with the expected length was generated, this amplicon was directly used for probe generation.
Northern Blot Analysis.
Digoxigenin (DIG)-labeled probes were generated by direct PCR labeling of the differential amplicons using the appropriate primers and conditions noted above except for dNTP concentrations as follows: 200 μM dATP, 200 μM dCTP, 200 μM dGTP, 190 μM dTTP, and 10 μM DIG-dUTP (Boehringer Mannheim). Northern blots were prepared with 20 μg of total RNA or with 2 μg of poly(A) RNA that had been electrophoresed through 1% agarose gels in the presence of 2.2 M formaldehyde. Equivalent loading of samples was verified by ethidium bromide staining of the ribosomal RNA bands or by using a DIG-labeled antisense RNA probe against the human heterogeneous nuclear ribonucleoprotein C1 as internal standard when poly(A) RNA was examined. The size of RNA was estimated by the DIG-labeled Molecular Weight Marker I (Boehringer Mannheim). Vacuum blotting (Appligene Oncor Trans DNA Express Vacuum Blotter) was used for transfer on positively charged nylon membranes (Boehringer Mannheim), which were then cross-linked under ultraviolet light (Stratagene UV Stratalinker 2400). Hybridization overnight was performed in DIG-Easy-Hyb (Boehringer Mannheim) at a probe concentration of 25 ng/ml or 100 ng/ml and at 50°C or at 65°C for DNA probes or RNA probes, respectively. Samples demonstrating differential expression were subcloned using the pCR-Script SK(+) cloning kit (Stratagene) and Northern blotting results were reconfirmed. Northern blots shown in the text are derived from these subclones.
Other Procedures.
DNA sequencing from the pCR cloning vector was performed with the Fidelity DNA Sequencing System (Appligene Oncor). Sequencing products were labeled with α-[35S]dATP and resolved on a 6% polyacrylamide/8 M urea sequencing gel. The GenBank database was searched (August 11, 1996) for homologous sequences, using the fasta computer program (20). The sequence of full-length h-sgk cDNA was obtained from the I.M.A.G.E. Consortium (clone ID 42669) from the American Type Culture Collection Special Collection of Human cDNA Clones (21). Gene database searches were performed at the European Molecular Biology Laboratory (Heidelberg) using the blast network service and—for protein alignments—the blitz server connected to the latest release of the SwissProt protein database.
RESULTS
Differential Gene Expression After Hypotonic, Isotonic, and Hypertonic Exposure of HepG2 Cells.
mRNA was isolated from HepG2 cells treated for 1 or 2 hr with hypotonic (hypotonic I: minus 100 mosmol/liter by removal of 50 mM NaCl and hypotonic II: minus 50 mosmol/liter by removal of 25 mM NaCl in comparison with the isotonic control medium), isotonic (with a total osmolarity of 290 mosmol/liter and a NaCl concentration of 114 mM) or hypertonic (plus 50 mosmol/liter by addition of 50 mM raffinose) medium. The mRNA was used as template for RAP–PCR using various random primers. The products of the RAP–PCR were displayed on denaturing polyacrylamide gels and run side-by-side for comparison. Several bands showed differential expression using various primers. Four differential bands from the RAP–PCR gel were analyzed further: two were revealed to be false positives upon Northern blot analysis, one was induced upon hypotonic and hypertonic conditions, but its sequence showed no similarities to any cloned gene. A band of about 500 bp, which showed increasing expression in cells treated with increasing extracellular osmolarity (hypotonic I–hypotonic II–isotonic–hypertonic), was purified from the gel and reamplified using primer RAP–A4. After labeling with DIG by PCR, a Northern blot containing RNA from a different preparation of cells treated for 2 hr with hypotonic I, isotonic, and hypertonic medium was probed with this amplicon to confirm its differential expression. A single transcript of about 2.6 kb strongly regulated by changing osmolarity could be observed (Fig. 1). Both directions of regulation occurred rapidly by changing the osmolarity: a down-regulation upon hypotonic treatment and an up-regulation upon hypertonic treatment.
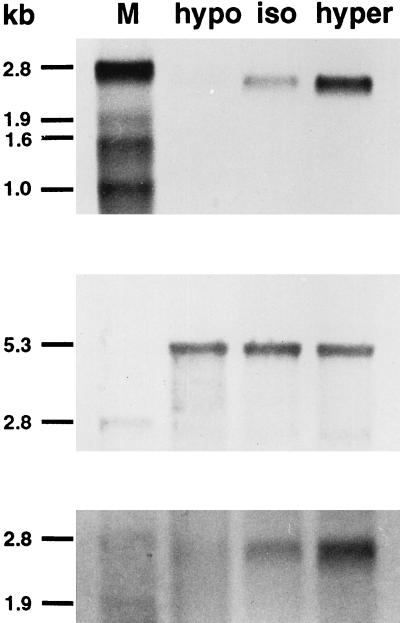
Figure 1.
Differential expression of the RAP–PCR product-encoded gene sequence. RNA (Northern) blot analysis confirming different transcript levels of the RAP–PCR product-encoded gene sequence by hypotonic (hypo, 190 mosmol/liter) and hypertonic (hyper, 390 mosmol/liter) treatment for 2 hr, in comparison with isotonic (iso, 290 mosmol/liter) cell culture conditions (Top). HepG2 mRNA (2.0 μg per lane) was hybridized with a 500 bp DIG-labeled cDNA probe generated by PCR reamplification of a differential band recovered from the RAP–PCR gel. Equal loading was confirmed by rehybridization with a DIG-labeled antisense RNA probe against the heterogeneous nuclear ribonucleoprotein C1 transcript (Middle). Hybridization with an antisense RNA probe against the 5′ end of the coding sequence of the full-length h-sgk clone yielded the same results as observed with the cDNA probe corresponding to the 3′ end of the coding sequence (Bottom). M, DIG-labeled RNA marker.
Cloning and Sequencing of the Differentially Regulated h-sgk Gene.
The 500-bp PCR product was subcloned into the pCR II vector and a new probe was generated using this construct to confirm identity between the original and subcloned DNA fragment. Rehybridization of a Northern blot with this probe gave identical results as with the original probe. Additionally, a Southern blot was performed with the new construct and hybridized with the original probe. A strong hybridization signal after two high-stringency washes further confirmed the sequence identity. Sequence analysis in both directions showed the presence of the arbitrary primer sequences at both ends of the amplicon. An amino acid sequence translated from one reading frame of the nucleotide sequence was found to have 95% identity with the carboxyl-terminal amino acid sequence of sgk (serum and glucocorticoid-regulated protein kinase), a novel member of the serine/threonine protein kinase family cloned from a rat mammary tumor cell line (22). Due to the distinct sequence similarity the designation h-sgk (human) was chosen for the clone under investigation. The GenBank database was searched for matching human sequences, using the fasta computer program. Several expressed sequence tag DNA sequences from the ATCC Special Collection of Human cDNA Clones (21) showed 100% sequence identity with parts of the h-sgk cDNA fragment. After multiple alignments of 30 different ATCC human cDNA clones with the rat sgk cDNA sequence (GenBank accession no. L01624L01624) and with the h-sgk DNA fragment, the I.M.A.G.E. Consortium construct with the clone ID 42669 from a human infant brain library was supposed to contain the complete coding sequence for h-sgk. Sequence analysis of this construct with confirmatory sequencing in sense and antisense direction revealed a cDNA sequence of about 2.4 kb. To verify the participation of full-length h-sgk in the described phenomenon, the 5′ end of the clone (nucleotides 1–285 of the coding sequence) was subcloned into the pCR II vector and a new probe was generated using this construct. Hybridization of a Northern blot with this probe gave identical results as with the original probe (Fig. 1). The longest open reading frame of the clone under investigation (1.3 kb) predicted a 431 amino-acid protein with an overall identity of 98% with the rat sgk protein.
Regulation of h-sgk Expression by Changes in Extracellular Osmolarity.
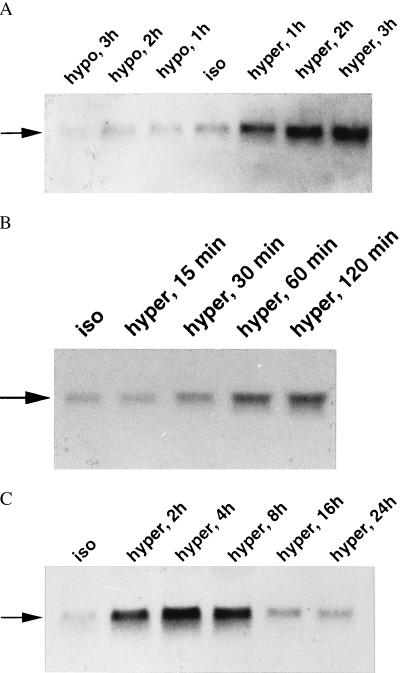
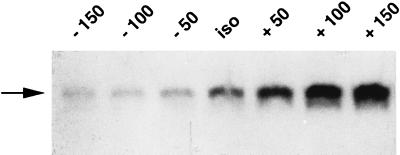
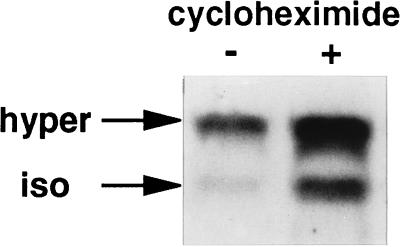
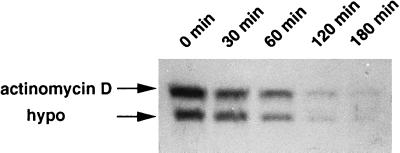
To characterize h-sgk transcript levels in response to osmotic changes, HepG2 cells were incubated in hypotonic (190 mosmol/liter), isotonic (290 mosmol/liter), and hypertonic (390 mosmol/liter) BME medium without FCS for various time periods. As shown in Fig. 2, h-sgk transcript levels were strongly raised within 60 min of exposure to hypertonicity with the first noticeable increase after 30 min and a maximum after 2–4 hr, suggesting that induction of h-sgk is an immediate response to this stimulus. Transcript levels continued to accumulate for 4–8 hr of exposure to hypertonic BME medium, with a subsequent decrease nearly to starting levels after 16–24 hr (Fig. 2). On the other hand, a rapid decrease of h-sgk transcript levels could be observed upon exposure to hypotonicity starting with the first visible decrease after 30 min and a maximum after 2 hr. Different degrees of osmotic changes (140, 190, 240, 290, 340, 390, and 440 mosmol/liter) were tested for an incubation period of 2 hr (Fig. 3). The increase of the osmolarity from 290 to 340 mosmol/liter, and its decrease from 290 to 240 mosmol/liter were sufficient to distinctly change the transcript levels of h-sgk, suggesting a transcriptional control mechanism very sensitive to extracellular osmolarity. The induction of h-sgk RNA was independent of de novo protein synthesis. A comparable increase of transcript levels upon addition of hypertonic BME medium could be observed in the absence and presence of the protein synthesis inhibitor cycloheximide (10 μg/ml), with higher h-sgk transcript levels throughout the presence of cycloheximide (Fig. 4). The rapid reduction in h-sgk transcript levels soon after lowering the extracellular osmolarity suggested that h-sgk mRNA had a particularly short half-life. To determine the rate of decay of h-sgk transcripts, HepG2 cells were treated with hypertonic medium (390 mosmol/liter) for 2 hr to maximally raise h-sgk transcript levels. Then to one part of the cells the RNA–polymerase inhibitor actinomycin D (5 μg/ml) was added and the other part of the cells was shifted into hypotonic medium (190 mosmol/liter). After various time periods, RNA was prepared and the transcript levels were compared between cells treated with actinomycin D and hypotonic medium (Fig. 5). Actinomycin D treatment caused a rapid decrease in h-sgk levels with an estimated half-life of approximately 30 min. Treatment of cells with hypotonic extracellular fluid proved equally effective as actinomycin D in respect to the down-regulation of h-sgk transcription.
Figure 2.
Changing h-sgk transcript levels in HepG2 cells following anisotonic exposure. (A) h-sgk transcript levels following hypertonic (hyper, 390 mosmol/liter) and hypotonic (hypo, 190 mosmol/liter) treatment for 1, 2, and 3 hr. (B) Increase of h-sgk after hypertonic treatment (hyper, 390 mosmol/liter) for 15, 30, 60, and 120 min. (C) Long-term regulation of h-sgk transcript levels by hypertonic treatment (hyper, 390 mosmol/liter) for 2, 4, 8, 16, and 24 hr. Cytoplasmic RNA (20 μg) from each time point was examined for h-sgk expression by Northern blot analysis as described. Iso, the basal expression under isotonic conditions (290 mosmol/liter). Arrow indicates the single 2.6-kb h-sgk transcript.
Figure 3.
h-sgk transcript levels in dependence of extracellular osmolarity. Prior to total cytoplasmic RNA isolation, HepG2 cells were kept in hypotonic (minus 50, minus 100, and minus 150 mosmol/liter) and hypertonic (+ 50, + 100, and + 150 mosmol/liter) solution for 2 hr. The basal expression under isotonic conditions (iso, 290 mosmol/liter) is shown in the middle lane. Arrow indicates the single 2.6-kb h-sgk transcript.
Figure 4.
Increase of h-sgk expression following hypertonic exposure in the absence and presence of cycloheximide. HepG2 cells were incubated with (+) or without (−) cycloheximide (10 μg/ml) 2 hr prior to transfer in hypertonic BME with (+) or without (−) cycloheximide for an additional 2 hr. Each slot of the gel was loaded two times with 20 μg of total cytoplasmic RNA with an interval of 15 min between the two loadings. The lower and the upper rows represent the expression of h-sgk under isotonic (iso, 290 mosmol/liter) or hypertonic conditions (hyper, 390 mosmol/liter), respectively.
Figure 5.
Decrease of h-sgk transcript levels upon hypotonic treatment or addition of actinomycin D. HepG2 cells were treated with hypertonic medium (390 mosmol/liter) 2 hr prior to the addition of actinomycin D (5 μg/ml) or transfer to hypotonic medium (hypo, 190 mosmol/liter). RNA was isolated at the time points indicated. Each slot of the gel was loaded two times with 20 μg of total cytoplasmic RNA with an interval of 15 min between the two loadings. The upper and the lower row represent the time course of the decrease of h-sgk transcript levels after addition of actinomycin D or after lowering the extracellular osmolarity, respectively.
Regulation of h-sgk Transcript Levels in Response to Isotonic Cell Volume Changes.
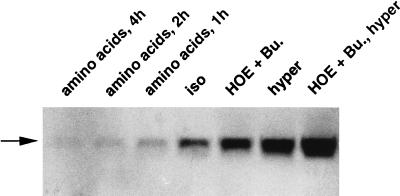
To discriminate between the effects of cell volume and those of ionic strength or osmolarity, two different maneuvers were performed to change the cell volume without changing the extracellular osmolarity. Isotonic cell shrinkage was achieved by blocking the NaCl/KCl cotransporter and the Na+/H+ exchanger with bumetanide and HOE694, respectively. Isotonic cell swelling was induced by addition of diverse amino acids after a 2-hr incubation period in BME medium without amino acids. As shown in Fig. 6, addition of bumetanide (10 μM) together with HOE694 (10 μM) increased within 2 hr h-sgk expression, which could be further stimulated by hypertonic treatment. Addition of an amino acid mixture in form of 1× BME amino acids (GIBCO/BRL) resulted in a marked and rapid decrease of h-sgk expression (Fig. 6). These data indicate that indeed cell volume rather than osmolarity modifies transcriptional regulation of h-sgk in HepG2 cells.
Figure 6.
Alteration of h-sgk expression by isotonic and anisotonic cell volume changes. HepG2 cells were treated with HOE694 (10 μM) and bumetanide (10 μM) (HOE + Bu.) or shifted in hypertonic medium without (hyper, 390 mosmol/liter) or with HOE694 (10 μM) and bumetanide (10 μM) (HOE + Bu., hyper) 2 hr prior to RNA isolation. 1× BME amino acid mixture was added for the indicated time periods. Twenty micrograms of total cytoplasmic RNA were subjected to Northern blot transfer and were examined for h-sgk expression. The basal expression under isotonic conditions without amino acids (iso, 290 mosmol/liter) is shown in the middle lane. Arrow indicates the single 2.6-kb h-sgk transcript.
To test if glucocorticoids and FCS influence transcription levels of h-sgk in HepG2 cells analogous to what was observed for sgk RNA in rat Con8.hd6 mammary tumor cells by Webster et al. (22), cells were treated with dexamethasone (1 μM) and with FCS (10%) for 2–12 hr. No changes in h-sgk transcript levels could be observed by Northern blot analysis after this pretreatment, suggesting different transcription regulatory mechanisms in human HepG2 cells (data not shown).
Regulation of sgk Transcript Levels by Anisotonicity in Madin–Darby–Canine Kidney (MDCK) Cells.
To investigate if the observed regulation is a peculiarity of HepG2 cells, MDCK cells were exposed to hypotonic (190 mosmol/liter) and hypertonic (390 mosmol/liter) BME medium 2 hr prior to RNA isolation. sgk transcripts with a length of about 2.6 kb could be identified even after several high-stringency wash steps with 0.5 × SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) at 65°C, suggesting distinct sequence homology of the sgk gene between different species. As a result, changing transcript levels comparable to that observed in HepG2 cells could be detected in the MDCK cells (Fig. 7).
Figure 7.
Altering sgk expression in MDCK cells following anisotonic exposure. sgk transcript levels are shown after hypotonic (hypo, 190 mosmol/liter) and hypertonic (hyper, 390 mosmol/liter) treatment for 2 hr, in comparison with isotonic (iso, 290 mosmol/liter) cell culture conditions.
Tissue-Specific Expression of h-sgk.
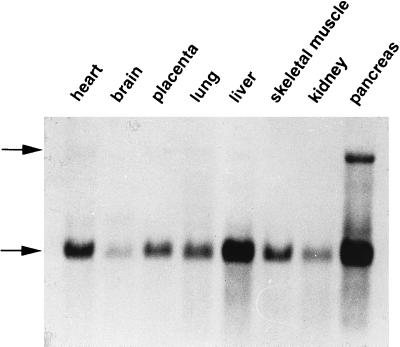
Since h-sgk cDNA had been isolated from a human hepatoma cell line, the tissue distribution of h-sgk mRNA in different human tissues was tested. To this purpose a premade Multiple Tissue Northern Blot (CLONTECH) was probed with the h-sgk DNA probe. As shown in Fig. 8, h-sgk expression has a distinct pattern of tissue specificity, with highest levels found in the pancreas, liver, and cardiac muscle. A somewhat lower expression was found in placenta, lung, and skeletal muscle. Low but detectable levels were found in brain and kidney. Surprisingly, a second transcript of 7 kb could be observed in nearly all tissues, being most prominent in human pancreas, which may represent another h-sgk mRNA species generated by alternative splicing or a gene homologous to h-sgk. This 7-kb transcript was not observed in any of the HepG2 Northern blots.
Figure 8.
Expression profile of h-sgk protein kinase transcripts in different human tissues. A Northern blot containing 2.0 μg poly(A)+ RNA from multiple human tissues was hybridized with a DIG-labeled h-sgk cDNA probe. A common 2.6-kb mRNA transcript abundant in pancreas, liver, and heart is indicated by the lower arrow. Upper arrow indicates a second 7.0-kb transcript most prominent in human pancreas.
DISCUSSION
In this study, one of the genes shown to be regulated on the transcriptional level during anisotonic and isotonic alterations of cell volume, h-sgk, encodes a putative human serine/threonine protein kinase with distinct sequence homology to sgk, which was recently isolated as a serum and glucocorticoid regulated gene from a rat mammary tumor cell line (22), as an injury-induced gene after central nervous system injury in rat brain (23) and as a testosterone- and follicle-stimulating hormone-induced gene in granulosa cells of rat ovary (24). The h-sgk gene encoded 49-kDa protein shares ≈98% overall sequence identity with the rat sgk protein with mainly conservative amino acid exchanges. It has ≈50% homology throughout its catalytic domain with several kinases of the second messenger family, including rac protein kinase, protein kinase C, ribosomal protein S6 kinase, and cAMP-dependent protein kinase (22, 25).
The expression level of the 2.6-kb h-sgk transcript in HepG2 cells was strongly regulated by anisotonic exposure of the cells in both directions. Increased transcript levels could be detected within 30 min of exposure to hypertonic extracellular fluid. This induction was independent of de novo protein synthesis, revealing this response as a primary one. Reduction of transcript levels occurred similarly within 30 min of exposure to hypotonic extracellular fluid. The time course of this down-regulation coincided perfectly with the decrease induced by actinomycin D suggesting a reduction of the h-sgk transcription rate. This reduced h-sgk transcription rate upon hypotonic treatment in combination with the short half-life could potently regulate the steady-state transcript levels of h-sgk RNA in HepG2 cells. Consistent with these properties of the h-sgk transcript are several AU-rich regions in the 3′ untranslated region of h-sgk, which are similar to those implicated in the rapid turnover of certain early response oncogenes (26) and cytokines (27).
Induction of isoosmotic cell volume changes similarly regulated the h-sgk expression. Cell shrinkage was achieved by inhibition of the major ion uptake mechanisms Na+/H+ exchanger (28–30) and the NaCl/KCl cotransporter (31, 32) by their specific blockers HOE694 and bumetanide, respectively. Cell swelling induced by the addition of amino acids is due to the concentrative uptake of amino acids by means of Na+-dependent amino acid transporters such as system A, N, and ASC in the plasma membrane of hepatocytes (33–40). Thus, the transcript levels were correlated with cell volume rather than osmolarity. Upon prolonged exposure to hypertonic extracellular fluid, the transcript levels increased within the first 30 min and remained enhanced for as long as 8 hr before gradually declining toward isotonic values. This long-lasting increase of transcript levels is in seeming contrast to the rapid time course of cell volume regulation. However, upon exposure to anisotonic extracellular fluid, liver cells do not fully regulate but remain slightly shrunken or swollen in hypertonic and hypotonic extracellular fluid, respectively, (ref. 8 and unpublished observations). These residual changes of cell volume may be the trigger for the expression of h-sgk. This does not necessarily mean that it is cell volume as such that regulates the transcription of h-sgk. Rather, during the anisotonic and isotonic alterations of cell volume, another cellular parameter could have been modified that in fact influences the expression of the kinase. Such a parameter could be macromolecular crowding, which has been invoked to strongly influence cellular function (41). Because the expression of the kinase is modified during isotonic and anisotonic cell volume changes, its actions are likely to mediate some alterations of cellular functions during changes of cell volume. However, the kinase does not necessarily contribute to cell volume regulation, i.e., stimulate cell volume regulatory ion transport or participate in regulation of cellular osmolyte metabolism.
Despite the striking sequence homology to the rat sgk protein, no parallels with the regulation as originally described by Webster et al. (22) could be observed in HepG2 cells. Neither serum (FCS) nor glucocorticoids (dexamethasone), which had been shown to strongly induce sgk transcription levels in rat mammary tumor cells regulated the h-sgk transcript levels in HepG2 cells, which may be due to different h-sgk promoter sequences in different cell types. Accordingly, cell volume or cell volume-related parameters may not be the only regulators of h-sgk expression and the stimuli modifying h-sgk expression may vary among different cell types. A cell volume dependent regulation similar to that observed in HepG2 cells was also detected in renal epithelial MDCK cells. Examination of the sgk 5′ flanking sequences in different cell types will be necessary to find regulatory elements that can account for the observed differences in the transcriptional control of sgk. Similar to the described glucocorticoid and serum induction profile of sgk in rat mammary tumor cells, h-sgk RNA was elevated within 30 min of hypertonic treatment. The half-life of the h-sgk transcripts in HepG2 cells was with 30 min just as short as the sgk half-life in rat mammary tumor cells determined by treatment with the RNA–polymerase inhibitor actinomycin D. Whereas most protein kinases are regulated in their catalytic activities by posttranslational mechanisms like interaction with regulatory proteins (42) or by phosphorylation (43), the sgk kinases seem to belong to a newly emerging subfamily of serine/threonine protein kinases, including snk, sgk, plk, and fnk, which are predominantly regulated at the transcriptional level by hormone- or mitogen-induced pathways (22, 25, 44–49). The rapid induction, the short half-life, and the particularly short regulatory sequence of the sgk kinases with no significant homologies to other regulatory sequences of protein kinases may serve as further indications in this context.
The h-sgk protein kinase transcript is expressed in all human tissues so far analyzed. The exceptionally high expression in human pancreas and liver may be a consequence of specialized secretory functions of these epithelial tissues. Activation of secretion is paralleled by loss of KCl and cell shrinkage in salivary glands, pancreatic acinar cells (50–52), and shark rectal glands (53). The cell shrinkage associated activation of the h-sgk transcription could explain the distinct h-sgk expression levels in these tissues. The strict and rapid transcriptional control of sgk expression by various stimuli like cell volume, glucocorticoids, growth factors (22), testosterone, and follicle-stimulating hormone (24) and cell injury (23) may account for the different expression levels with respect to human tissues in a selection of various rat tissues (22, 23). There, the highest expression levels were found in kidney and intestine in one study (23) and in thymus, ovary, and lung in another study (22).
Protein phosphorylation is a rapid and reversible means of transducing signals from the extracellular environment that lead to pleiotropic cellular responses (54). The h-sgk protein kinase, by phosphorylation of specific target proteins, may be one of the secondary mediators of a subset of cellular responses to cell shrinkage and cell swelling and may represent, at least in part, a hitherto missing link between cell hydration and cell function.
Acknowledgments
We are indebted to Mrs. Elke Sailer for her expert technical assistance and to Mrs. Uta Hamacher for her expertise in all cell culture tasks. This work was supported by Deutsche Forschungsgemeinschaft Grant Nr. 315/4-2
ABBREVIATIONS
- DIG
digoxigenin
- MDCK
Madin–Darby–Canine Kidney
Footnotes
Data deposition: The h-sgk cDNA sequence reported in this paper has been deposited in the GenBank database (accession no. Y10032Y10032).
To whom reprint requests should be addressed at: Institut für Physiologie der Eberhard-Karls-Universität, Gmelinstrasse 5, 72076 Tübingen, Germany. e-mail: 106013.3522@compuserve.com.
References
- 1.Lang, F., Busch, G. L. & Völkl, H. (1997) Cell. Physiol. Biochem., in press. [DOI] [PubMed]
- 2.Brown G C. J Theor Biol. 1991;153:195–203. doi: 10.1016/s0022-5193(05)80422-9. [DOI] [PubMed] [Google Scholar]
- 3.Fulton A B. Cell. 1982;30:345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- 4.Garner M M, Burg M B. Am J Physiol. 1994;266:C877–C892. doi: 10.1152/ajpcell.1994.266.4.C877. [DOI] [PubMed] [Google Scholar]
- 5.Minton A P. In: Cellular and Molecular Physiology of Cell Volume Regulation. Strange K, editor. Boca Raton, FL: CRC; 1994. pp. 181–190. [Google Scholar]
- 6.Parker J C, Colclasure G C. Mol Cell Biochem. 1992;114:9–11. doi: 10.1007/BF00240291. [DOI] [PubMed] [Google Scholar]
- 7.Burg M B. Am J Physiol. 1995;268:F983–F996. doi: 10.1152/ajprenal.1995.268.6.F983. [DOI] [PubMed] [Google Scholar]
- 8.Häussinger D, Lang F, Gerok W. Am J Physiol. 1994;267:E343–E355. doi: 10.1152/ajpendo.1994.267.3.E343. [DOI] [PubMed] [Google Scholar]
- 9.Häussinger D, Lang F. Biochim Biophys Acta. 1991;1071:331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- 10.Häussinger D, Roth E, Lang F, Gerok W. Lancet. 1993;341:1330–1332. doi: 10.1016/0140-6736(93)90828-5. [DOI] [PubMed] [Google Scholar]
- 11.Newsome W P, Warskulat U, Noe B, Wettstein M, Stoll B, Gerok W, Häussinger D. Biochem J. 1994;304:555–560. doi: 10.1042/bj3040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundgreen D V. J Biol Chem. 1992;267:6841–6847. [PubMed] [Google Scholar]
- 13.Finkenzeller G, Newsome W P, Lang F, Häussinger D. FEBS Lett. 1994;340:163–166. doi: 10.1016/0014-5793(94)80129-0. [DOI] [PubMed] [Google Scholar]
- 14.Häussinger D, Lang F. Trends Pharmacol Sci. 1992;13:371–373. doi: 10.1016/0165-6147(92)90114-l. [DOI] [PubMed] [Google Scholar]
- 15.Häussinger D, Lang F, Bauers K, Gerok W. Eur J Biochem. 1990;188:689–695. doi: 10.1111/j.1432-1033.1990.tb15451.x. [DOI] [PubMed] [Google Scholar]
- 16.Hallbrucker C, Ritter M, Lang F, Gerok W, Häussinger D. Eur J Biochem. 1993;211:449–458. doi: 10.1111/j.1432-1033.1993.tb17570.x. [DOI] [PubMed] [Google Scholar]
- 17.Saha N, Schreiber R, vom Dahl S, Lang F, Gerok W, Häussinger D. Biochem J. 1993;296:701–707. doi: 10.1042/bj2960701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClelland M, Ralph D, Cheng R, Welsh J. Nucleic Acids Res. 1994;22:4419–4431. doi: 10.1093/nar/22.21.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanguinetti C J, Neto E D, Simpson A J G. Biotechniques. 1994;17:914–921. [PubMed] [Google Scholar]
- 20.Pearson W R, Lipman D J. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lennon G, Auffray C, Polymeropoulos M, Soares M B. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- 22.Webster M K, Goya L, Ge Y, Maiyar A C, Firestone G L. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imaizumi K, Tsuda M, Wanaka A, Tohyama M, Takagi T. Mol Brain Res. 1994;26:189–196. doi: 10.1016/0169-328x(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Richards J S, Fitzpatrick S L, Clemens J W, Morris J K, Alliston T, Sirois J. Recent Prog Horm Res. 1995;50:223–254. doi: 10.1016/b978-0-12-571150-0.50014-7. [DOI] [PubMed] [Google Scholar]
- 25.Webster M K, Goya L, Firestone G L. J Biol Chem. 1993;268:11482–11485. [PubMed] [Google Scholar]
- 26.Hargrove J L, Schmidt F H. FASEB J. 1989;3:2360–2370. doi: 10.1096/fasebj.3.12.2676679. [DOI] [PubMed] [Google Scholar]
- 27.Shaw G, Kamen R. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 28.Demaurex N, Grinstein S. J Exp Biol. 1994;196:389–404. doi: 10.1242/jeb.196.1.389. [DOI] [PubMed] [Google Scholar]
- 29.Grinstein S, Woodside M, Sardet C, Pouyssegur J, Rotin D. J Biol Chem. 1992;267:23823–23828. [PubMed] [Google Scholar]
- 30.Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowsky J. J Biol Chem. 1994;269:23544–23552. [PubMed] [Google Scholar]
- 31.Gamba G, Saltzberg S N, Lombardi M, Miyanoshita A, Lytton J, Hedinger M A, Brenner B M, Hebert S C. Proc Natl Acad Sci USA. 1993;90:2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J C, Lytle C, Zhu T T, Payne J A, Benz E, Forbush B. Proc Natl Acad Sci USA. 1994;91:2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakker-Grunwald T. Biochim Biophys Acta. 1983;731:239–242. doi: 10.1016/0005-2736(83)90014-7. [DOI] [PubMed] [Google Scholar]
- 34.Baquet A, Lavoinne A, Hue L. Biochem J. 1991;273:57–62. doi: 10.1042/bj2730057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bear C E, Peterson O H. Pflügers Arch. 1987;410:342–344. doi: 10.1007/BF00580288. [DOI] [PubMed] [Google Scholar]
- 36.Cohen B J, Lechene C. Am J Physiol. 1990;258:C24–C29. doi: 10.1152/ajpcell.1990.258.1.C24. [DOI] [PubMed] [Google Scholar]
- 37.Fosse M, Berg T O, O’Reilly D S, Seglen P O. Eur J Biochem. 1995;230:17–24. doi: 10.1111/j.1432-1033.1995.0017i.x. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen L O. Am J Physiol. 1986;251:G575–G584. doi: 10.1152/ajpgi.1986.251.5.G575. [DOI] [PubMed] [Google Scholar]
- 39.Wang K, Wondergem R. J Membr Biol. 1993;135:237–244. doi: 10.1007/BF00211095. [DOI] [PubMed] [Google Scholar]
- 40.Wettstein M, vom Dahl S, Lang F, Gerok W, Häussinger D. Biol Chem Hoppe-Seyler. 1990;371:493–501. doi: 10.1515/bchm3.1990.371.1.493. [DOI] [PubMed] [Google Scholar]
- 41.Garner M M, Burg M B. Am J Physiol. 1994;266:C877–C892. doi: 10.1152/ajpcell.1994.266.4.C877. [DOI] [PubMed] [Google Scholar]
- 42.Morrison D. Science. 1994;266:56–57. doi: 10.1126/science.7939645. [DOI] [PubMed] [Google Scholar]
- 43.Hunter T, Karin M. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 44.Donohue P J, Alberts G F, Guo Y, Winkles J A. J Biol Chem. 1995;270:10351–10357. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 45.Clay F J, McEwen S J, Bertoncello I, Wilks A F, Dunn A R. Proc Natl Acad Sci USA. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golsteyn R M, Schultz S J, Bartek J, Ziemiecki A, Ried T, Nigg E A. J Cell Sci. 1994;107:1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 47.Hamanaka R, Maloid S, Smith M R, O’Connell C D, Longo D L, Ferris D K. Cell Growth Differ. 1994;5:249–257. [PubMed] [Google Scholar]
- 48.Holtrich U, Wolf G, Brauninger A, Karn T, Bohme B, Rubsamen W H, Strebhardt K. Proc Natl Acad Sci USA. 1994;91:1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simmons D L, Neel B G, Stevens R, Evett G, Erikson R L. Mol Cell Biol. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen O H, Gallacher D V. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- 51.Petersen O H. Comp Biochem Physiol A. 1988;90:717–721. doi: 10.1016/0300-9629(88)90689-5. [DOI] [PubMed] [Google Scholar]
- 52.Foskett J K, Melvin J E. Science. 1989;244:1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- 53.Greger R, Gögelein H, Schlatter E. Comp Biochem Physiol A. 1988;90:733–739. doi: 10.1016/0300-9629(88)90692-5. [DOI] [PubMed] [Google Scholar]
- 54.Witters L A. Curr Opin Cell Biol. 1990;2:212–220. doi: 10.1016/0955-0674(90)90009-4. [DOI] [PubMed] [Google Scholar]