Abstract
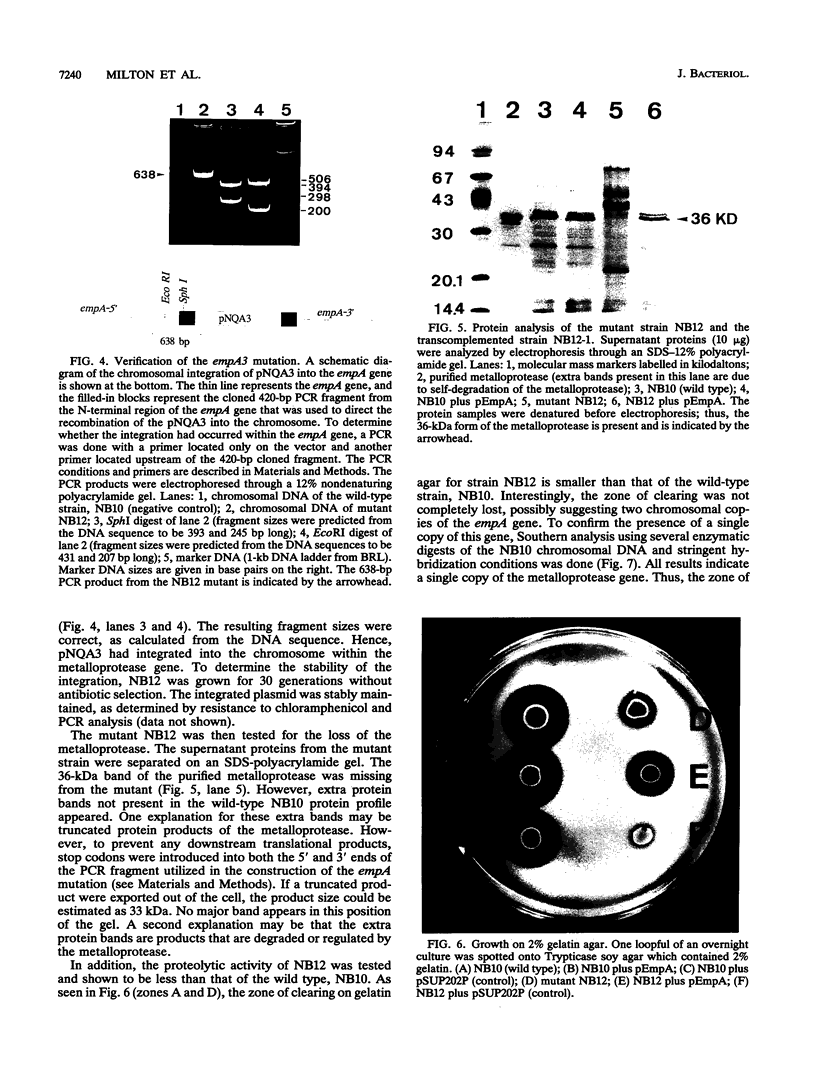
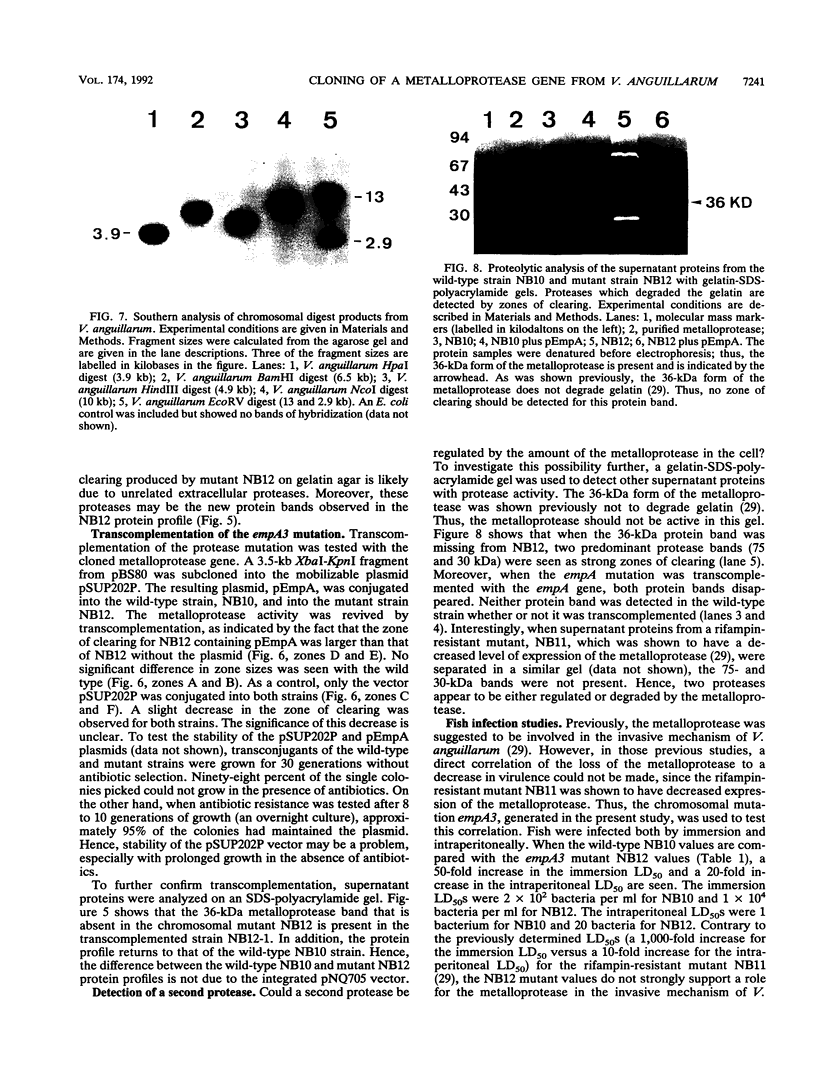
Genetic evidence has previously suggested that a zinc metalloprotease is involved in the invasive mechanism of the fish pathogen Vibrio anguillarum NB10. In this study, the metalloprotease gene was cloned and sequenced. The sequence encodes a polypeptide (611 amino acids) that contains a putative signal sequence followed by a large leader sequence and the mature protein (44.6 kDa). Since the purified protein has a molecular mass of 36 kDa instead of the predicted 44.6 kDa, the mature protein is most likely processed a third time. Comparative analyses of the protein sequence showed high homologies to other bacterial metalloproteases within the zinc-binding and active-site regions. The Vibrio cholerae hemagglutinin/protease and the Pseudomonas aeruginosa elastase were exceptions in that the homology extended throughout the entire putative preproprotein. A chromosomal metalloprotease mutant was made via the integration of foreign DNA into the protease gene. This mutant did not secrete the metalloprotease, as determined by sodium dodecyl sulfate (SDS)-polyacrylamide protein analysis and by growth on gelatin agar. Transcomplementation of the chromosomal mutation revived the secretion of the metalloprotease and its activity on gelatin agar. Interestingly, when supernatant proteins were analyzed by gelatin-SDS-polyacrylamide electrophoresis, two different proteases (75 and 30 kDa) were detected in the mutant strain but not in the transcomplemented strain or the wild-type strain. Moreover, fish infection studies were done, and implications for the role of the metalloprotease in the virulence mechanism of V. anguillarum are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actis L. A., Fish W., Crosa J. H., Kellerman K., Ellenberger S. R., Hauser F. M., Sanders-Loehr J. Characterization of anguibactin, a novel siderophore from Vibrio anguillarum 775(pJM1). J Bacteriol. 1986 Jul;167(1):57–65. doi: 10.1128/jb.167.1.57-65.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actis L. A., Potter S. A., Crosa J. H. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J Bacteriol. 1985 Feb;161(2):736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Actis L. A., Tolmasky M. E., Farrell D. H., Crosa J. H. Genetic and molecular characterization of essential components of the Vibrio anguillarum plasmid-mediated iron-transport system. J Biol Chem. 1988 Feb 25;263(6):2853–2860. [PubMed] [Google Scholar]
- David V. A., Deutch A. H., Sloma A., Pawlyk D., Ally A., Durham D. R. Cloning, sequencing and expression of the gene encoding the extracellular neutral protease, vibriolysin, of Vibrio proteolyticus. Gene. 1992 Mar 1;112(1):107–112. doi: 10.1016/0378-1119(92)90310-l. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Chang Y., Häse C. C. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992 Feb;60(2):472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. R. Pseudomonas aeruginosa elastase and elastolysis revisited: recent developments. Mol Microbiol. 1991 Oct;5(10):2315–2321. doi: 10.1111/j.1365-2958.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Gray L. D., Kreger A. S. Rabbit corneal damage produced by Pseudomonas aeruginosa infection. Infect Immun. 1975 Aug;12(2):419–432. doi: 10.1128/iai.12.2.419-432.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L., Kreger A. Microscopic characterization of rabbit lung damage produced by Pseudomonas aeruginosa proteases. Infect Immun. 1979 Jan;23(1):150–159. doi: 10.1128/iai.23.1.150-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., Iglewski B. H. Isolation and characterization of alkaline protease-deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect Immun. 1984 Mar;43(3):1058–1063. doi: 10.1128/iai.43.3.1058-1063.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J Bacteriol. 1991 Jun;173(11):3311–3317. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Comparison of the Vibrio cholerae hemagglutinin/protease and the Pseudomonas aeruginosa elastase. Infect Immun. 1990 Dec;58(12):4011–4015. doi: 10.1128/iai.58.12.4011-4015.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen M. G., Hoffman P. S. Characterization of a Legionella pneumophila extracellular protease exhibiting hemolytic and cytotoxic activities. Infect Immun. 1989 Mar;57(3):732–738. doi: 10.1128/iai.57.3.732-738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M. H., Kreger A. S. Production and partial characterization of an elastolytic protease of Vibrio vulnificus. Infect Immun. 1985 Nov;50(2):534–540. doi: 10.1128/iai.50.2.534-540.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M. H., Kreger A. S. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J Gen Microbiol. 1987 Jul;133(7):1783–1791. doi: 10.1099/00221287-133-7-1783. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Griffin O. K. Cornea-damaging proteases of Serratia marcescens. Invest Ophthalmol. 1975 Mar;14(3):190–198. [PubMed] [Google Scholar]
- Matthews B. W., Weaver L. H., Kester W. R. The conformation of thermolysin. J Biol Chem. 1974 Dec 25;249(24):8030–8044. [PubMed] [Google Scholar]
- Mengaud J., Geoffroy C., Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991 Mar;59(3):1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N., Miyoshi S., Sugiyama K., Suzuki Y., Furuta H., Shinoda S. Activation of the plasma kallikrein-kinin system by Vibrio vulnificus protease. Infect Immun. 1987 Aug;55(8):1936–1939. doi: 10.1128/iai.55.8.1936-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina Y., Miyoshi S., Nagase A., Shinoda S. Significant role of an exocellular protease in utilization of heme by Vibrio vulnificus. Infect Immun. 1992 May;60(5):2128–2132. doi: 10.1128/iai.60.5.2128-2132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norqvist A., Hagström A., Wolf-Watz H. Protection of rainbow trout against vibriosis and furunculosis by the use of attenuated strains of Vibrio anguillarum. Appl Environ Microbiol. 1989 Jun;55(6):1400–1405. doi: 10.1128/aem.55.6.1400-1405.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norqvist A., Norrman B., Wolf-Watz H. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect Immun. 1990 Nov;58(11):3731–3736. doi: 10.1128/iai.58.11.3731-3736.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlner J., Halter R., Beyreuther K., Meyer T. F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. 1987 Jan 29-Feb 4Nature. 325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- Raveneau J., Geoffroy C., Beretti J. L., Gaillard J. L., Alouf J. E., Berche P. Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect Immun. 1992 Mar;60(3):916–921. doi: 10.1128/iai.60.3.916-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. C., Merkel J. R. Collagenolytic activity of Vibrio vulnificus: potential contribution to its invasiveness. Infect Immun. 1982 Mar;35(3):1155–1156. doi: 10.1128/iai.35.3.1155-1156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto L., Shuman H. A. The Legionella pneumophila major secretory protein, a protease, is not required for intracellular growth or cell killing. Infect Immun. 1990 Aug;58(8):2585–2592. doi: 10.1128/iai.58.8.2585-2592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer M. M., Flaherty K. M., McKay D. B. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-A resolution. J Biol Chem. 1991 Feb 15;266(5):2864–2871. doi: 10.2210/pdb1ezm/pdb. [DOI] [PubMed] [Google Scholar]
- Tolmasky M. E., Crosa J. H. Molecular cloning and expression of genetic determinants for the iron uptake system mediated by the Vibrio anguillarum plasmid pJM1. J Bacteriol. 1984 Dec;160(3):860–866. doi: 10.1128/jb.160.3.860-866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. A., Potter S. A., Crosa J. H. Iron uptake system medicated by Vibrio anguillarum plasmid pJM1. J Bacteriol. 1983 Nov;156(2):880–887. doi: 10.1128/jb.156.2.880-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida N., Uozumi T., Beppu T. Specific excretion of Serratia marcescens protease through the outer membrane of Escherichia coli. J Bacteriol. 1986 Jun;166(3):937–944. doi: 10.1128/jb.166.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]