Abstract
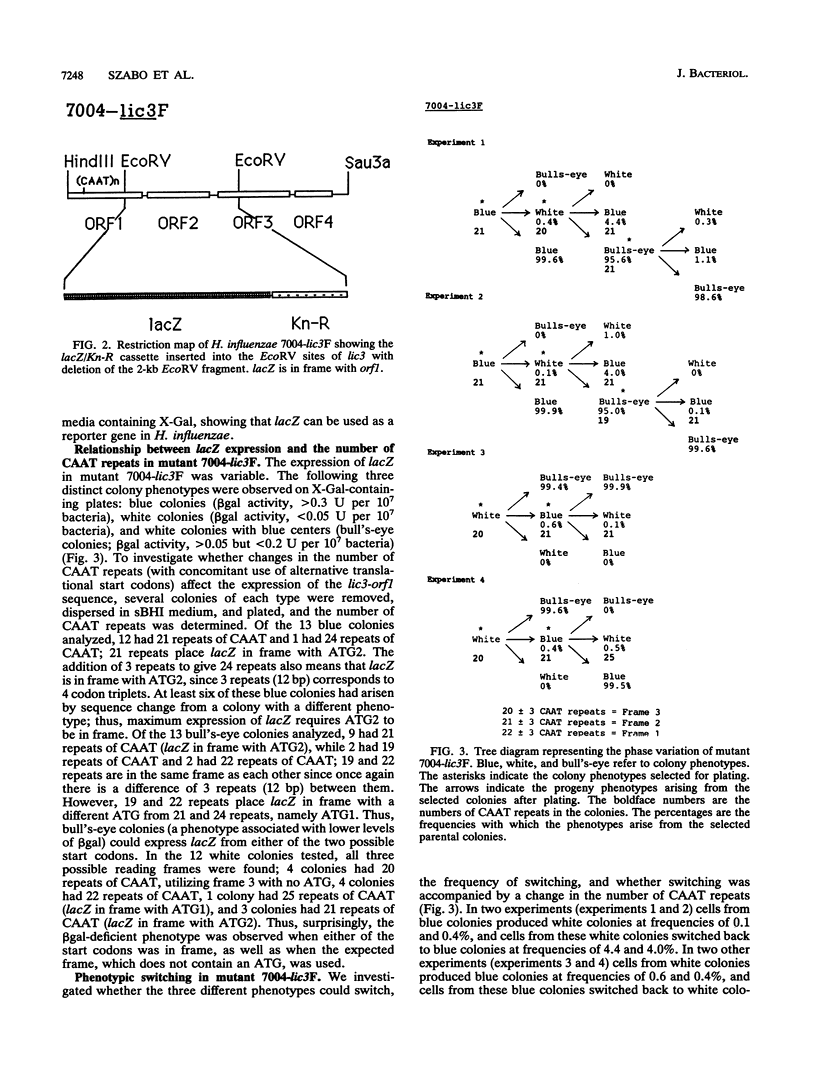
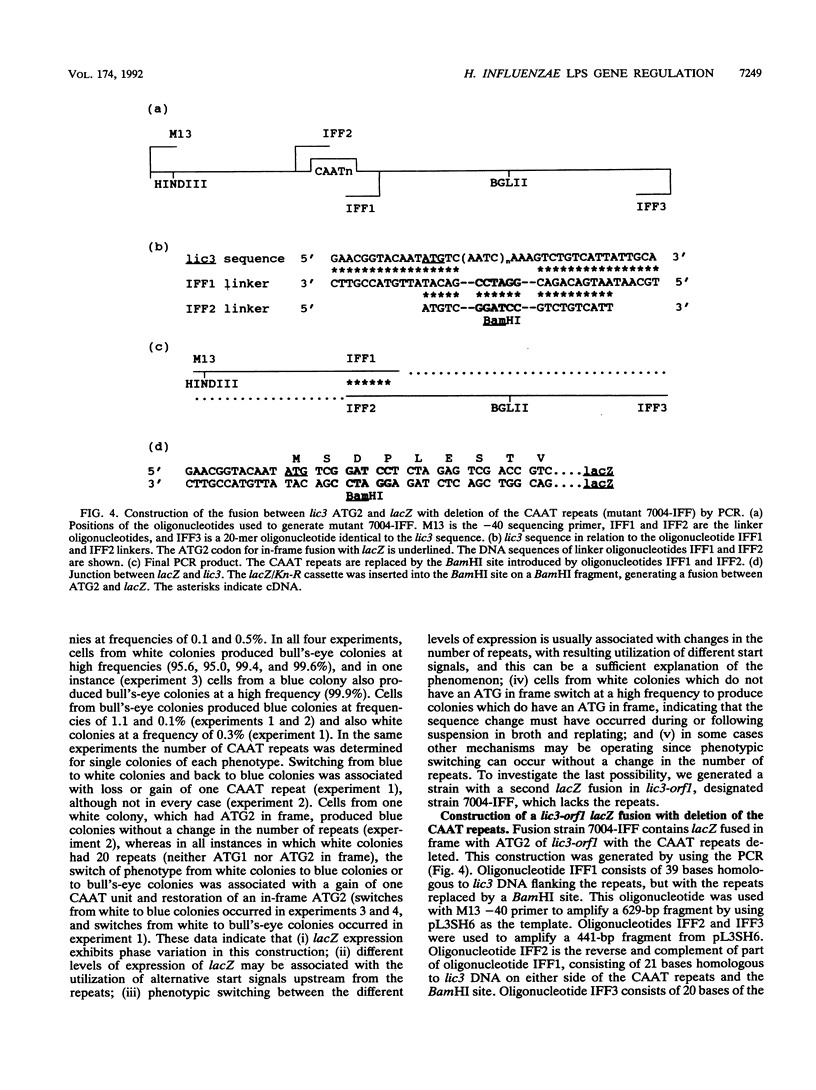
The lic3 locus of Haemophilus influenzae consists of four open reading frames. The derived amino acid sequences of orf2 and orf4 exhibit homology to Escherichia coli GalE and AdK, respectively. The functions of orf1 and orf3 remain unknown. orf1 contains multiple tandem repeats of the tetrameric DNA sequence CAAT near the 5' end. Two possible translational starts (ATG1 and ATG2) lie upstream. We have used lacZ fusions to investigate whether changes in the number of CAAT repeats in conjunction with differential usage of the upstream frames control the expression of lic3-orf1. Phase-variable expression of lacZ was observed for individual colonies and could be related to variable numbers of CAAT repeats. Of the three possible upstream frames, only one, containing the more downstream of the two possible ATG start codons (ATG2), is used for strong expression of lacZ. Utilization of the more upstream ATG (ATG1) or ATG2 was observed with medium-level expression, while utilization of any of the three possible frames was observed when lacZ was expressed at low to undetectable levels, indicating that other mechanisms may affect expression. To investigate this, lacZ was fused in frame with ATG2 of lic3-orf1, with concomitant deletion of the repeats. Phase-variable expression was still observed, supporting the view that an alternative level of control operates in conjunction with the repeat mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belland R. J., Morrison S. G., van der Ley P., Swanson J. Expression and phase variation of gonococcal P.II genes in Escherichia coli involves ribosomal frameshifting and slipped-strand mispairing. Mol Microbiol. 1989 Jun;3(6):777–786. doi: 10.1111/j.1365-2958.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Cope L. D., Yogev R., Mertsola J., Latimer J. L., Hanson M. S., McCracken G. H., Jr, Hansen E. J. Molecular cloning of a gene involved in lipooligosaccharide biosynthesis and virulence expression by Haemophilus influenzae type B. Mol Microbiol. 1991 May;5(5):1113–1124. doi: 10.1111/j.1365-2958.1991.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Dealler S. F., Foweraker J., Lawson P., Fortune S. Provisional identification of Haemophilus influenzae from sputum cultures within 1 h by rapid enzyme tests. J Med Microbiol. 1991 Jul;35(1):49–52. doi: 10.1099/00222615-35-1-49. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics. 1990 Sep;126(1):5–16. doi: 10.1093/genetics/126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Maskell D. J., Szabo M. J., Butler P. D., Williams A. E., Moxon E. R. Molecular analysis of a complex locus from Haemophilus influenzae involved in phase-variable lipopolysaccharide biosynthesis. Mol Microbiol. 1991 May;5(5):1013–1022. doi: 10.1111/j.1365-2958.1991.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Gibbs C. P., Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- Petersen G. B., Stockwell P. A., Hill D. F. Messenger RNA recognition in Escherichia coli: a possible second site of interaction with 16S ribosomal RNA. EMBO J. 1988 Dec 1;7(12):3957–3962. doi: 10.1002/j.1460-2075.1988.tb03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Cannon J. G., So M. Phase and antigenic variation of pili and outer membrane protein II of Neisseria gonorrhoeae. J Infect Dis. 1986 Feb;153(2):196–201. doi: 10.1093/infdis/153.2.196. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Lindberg A. A., Manning E. J., Hansen E. J., Moxon E. R. Identification of a chromosomal locus for expression of lipopolysaccharide epitopes in Haemophilus influenzae. Infect Immun. 1989 Oct;57(10):3045–3052. doi: 10.1128/iai.57.10.3045-3052.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser J. N., Love J. M., Moxon E. R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989 Nov 17;59(4):657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Maskell D. J., Butler P. D., Lindberg A. A., Moxon E. R. Characterization of repetitive sequences controlling phase variation of Haemophilus influenzae lipopolysaccharide. J Bacteriol. 1990 Jun;172(6):3304–3309. doi: 10.1128/jb.172.6.3304-3309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zamze S. E., Moxon E. R. Composition of the lipopolysaccharide from different capsular serotype strains of Haemophilus influenzae. J Gen Microbiol. 1987 Jun;133(6):1443–1451. doi: 10.1099/00221287-133-6-1443. [DOI] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Hopman C., Zanen H. C. Homogeneity of cell envelope protein subtypes, lipopolysaccharide serotypes, and biotypes among Haemophilus influenzae type b from patients with meningitis in The Netherlands. J Infect Dis. 1983 Jul;148(1):75–81. doi: 10.1093/infdis/148.1.75. [DOI] [PubMed] [Google Scholar]


