Abstract
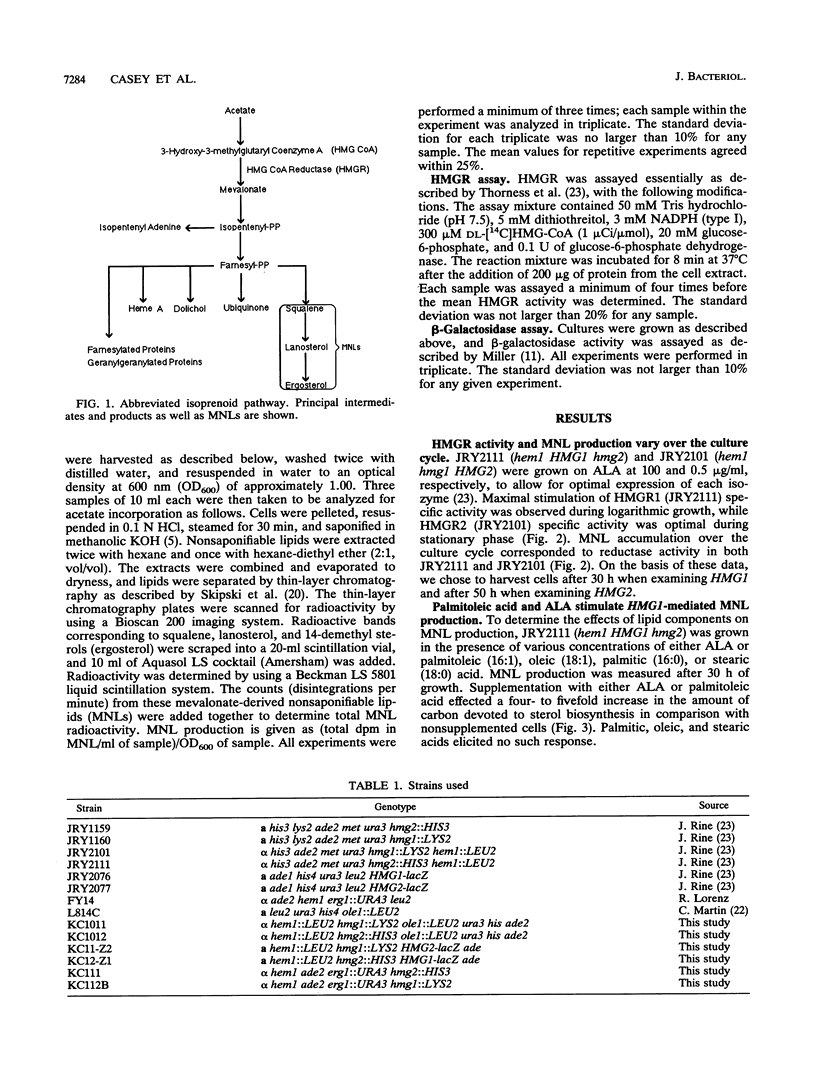
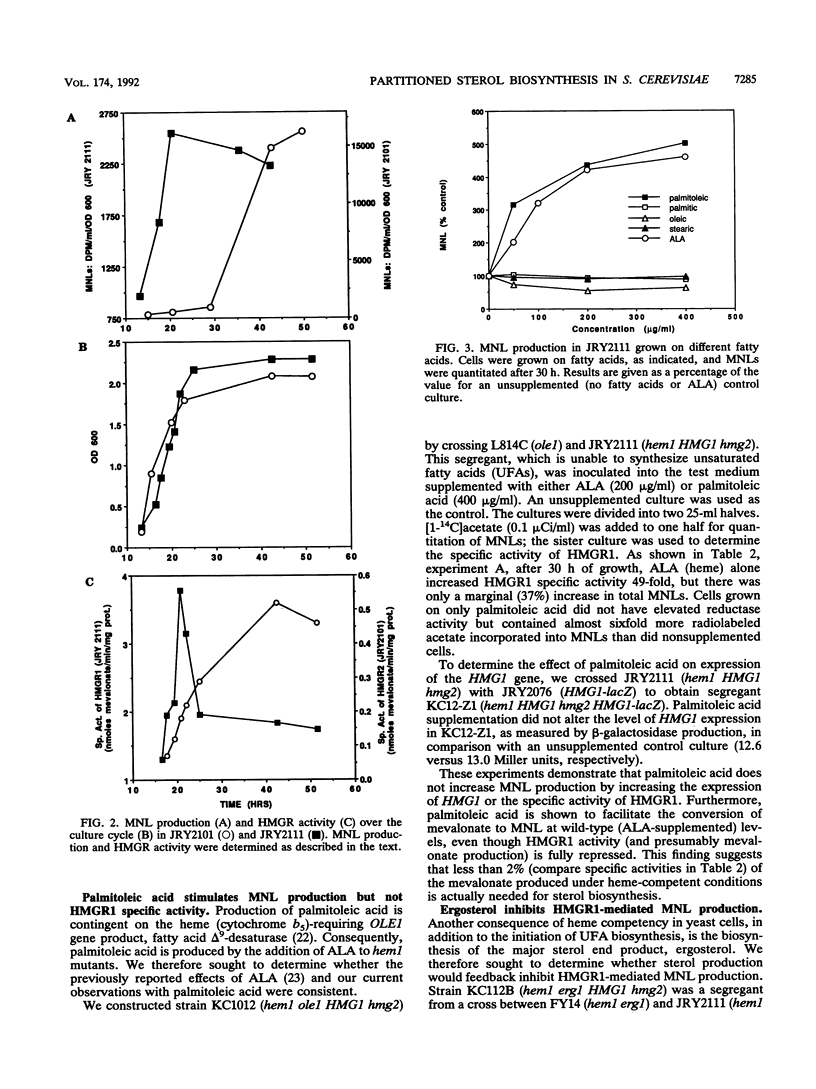
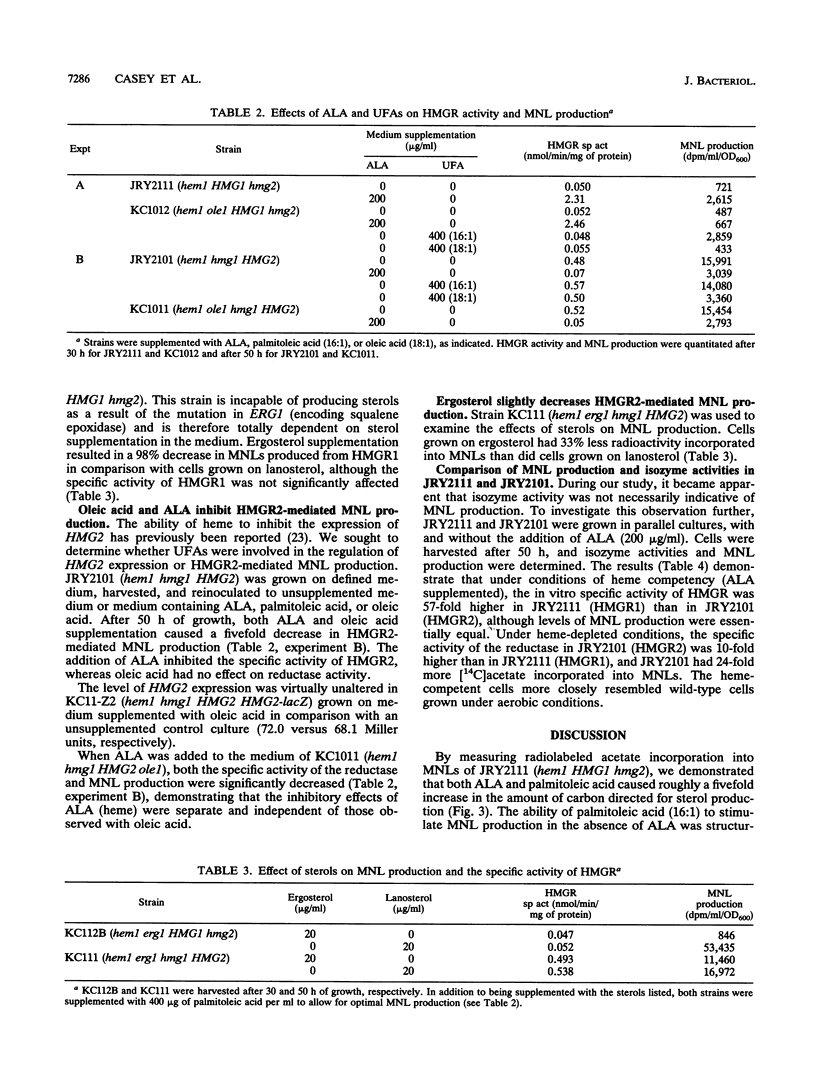
Using yeast strains with null mutations in structural genes which encode delta-aminolevulinic acid synthetase (HEM1), isozymes of 3-hydroxy-3-methylglutaryl coenzyme A (HMG1 and HMG2), squalene epoxidase (ERG1), and fatty acid delta 9-desaturase (OLE1), we were able to determine the effect of hemes, sterols, and unsaturated fatty acids on both sterol production and the specific activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) in Saccharomyces cerevisiae. We found that the HMGR isozymes direct essentially equal amounts of carbon to the biosynthesis of sterols under heme-competent conditions, despite a huge disparity (57-fold) in the specific activities of the reductases. Our results demonstrate that palmitoleic acid (16:1) acts as a rate-limiting positive regulator and that ergosterol acts as a potent inhibitor of sterol production in strains which possess only the HMGR1 isozyme (HMG1 hmg2). In strains which contain only the HMGR2 isozyme (hmg1 HMG2), sterol production was inhibited by oleic acid (18:1) and to a lesser degree by ergosterol. The specific activities of the two reductases (HMGR1 and HMGR2) were found to be differentially regulated by hemes but not by ergosterol, palmitoleic acid, or oleic acid. The disparate effects of unsaturated fatty acids and sterols on these strains lead us to consider the possibility of separate, compartmentalized isoprenoid pathways in S. cerevisiae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M., Downing J. F. Genetic and biochemical aspects of yeast sterol regulation involving 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Gen Microbiol. 1981 Aug;125(2):415–420. doi: 10.1099/00221287-125-2-415. [DOI] [PubMed] [Google Scholar]
- Basson M. E., Thorsness M., Rine J. Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5563–5567. doi: 10.1073/pnas.83.15.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K. E. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14(1):47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Casey W. M., Burgess J. P., Parks L. W. Effect of sterol side-chain structure on the feed-back control of sterol biosynthesis in yeast. Biochim Biophys Acta. 1991 Feb 5;1081(3):279–284. doi: 10.1016/0005-2760(91)90283-n. [DOI] [PubMed] [Google Scholar]
- Lorenz R. T., Parks L. W. Regulation of ergosterol biosynthesis and sterol uptake in a sterol-auxotrophic yeast. J Bacteriol. 1987 Aug;169(8):3707–3711. doi: 10.1128/jb.169.8.3707-3711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C., Rodriguez R. J., Parks L. W. Modulation of yeast plasma membrane composition of a yeast sterol auxotroph as a function of exogenous sterol. Arch Biochem Biophys. 1985 Aug 1;240(2):530–538. doi: 10.1016/0003-9861(85)90059-1. [DOI] [PubMed] [Google Scholar]
- Mercer E. I. Sterol biosynthesis inhibitors: their current status and modes of action. Lipids. 1991 Aug;26(8):584–597. doi: 10.1007/BF02536422. [DOI] [PubMed] [Google Scholar]
- Olson R. E., Rudney H. Biosynthesis of ubiquinone. Vitam Horm. 1983;40:1–43. doi: 10.1016/s0083-6729(08)60431-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. Application of high-performance liquid chromatographic separation of free sterols to the screening of yeast sterol mutants. Anal Biochem. 1982 Jan 1;119(1):200–204. doi: 10.1016/0003-2697(82)90686-8. [DOI] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- STARR P. R., PARKS L. W. Some factors affecting sterol formation in Saccharomyces cerevisiae. J Bacteriol. 1962 May;83:1042–1046. doi: 10.1128/jb.83.5.1042-1046.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Servouse M., Karst F. Regulation of early enzymes of ergosterol biosynthesis in Saccharomyces cerevisiae. Biochem J. 1986 Dec 1;240(2):541–547. doi: 10.1042/bj2400541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Rodwell V. W. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol synthesis. J Biol Chem. 1971 May 25;246(10):3210–3216. [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Sullivan R. C., Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1965 Oct 4;106(2):386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J Biol Chem. 1989 Oct 5;264(28):16537–16544. [PubMed] [Google Scholar]
- Thorsness M., Schafer W., D'Ari L., Rine J. Positive and negative transcriptional control by heme of genes encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Dec;9(12):5702–5712. doi: 10.1128/mcb.9.12.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]



