Abstract
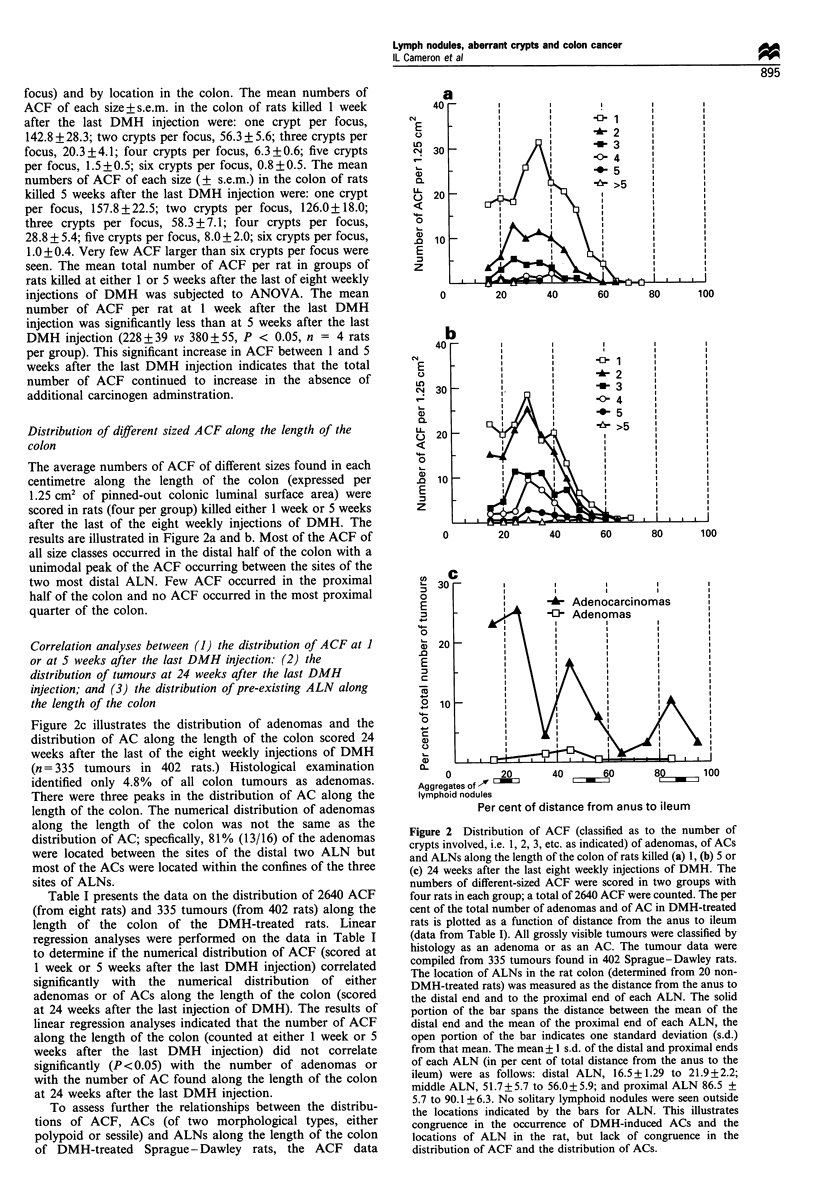
Sprague-Dawley rats were given eight weekly subcutaneous injections of 1,2-dimethylhydrazine (DMH) or of vehicle then were sacrificed at 1, 5 or 24 weeks after the last injection of DMH. The locations of pre-existing aggregates of lymphoid nodules (ALNs), the location and multiplicity (size) of aberrant crypt foci (ACF), and the locations of tumours in the colon were determined. A trimodal distribution of pre-existing ALNs along the length of the colon was significantly correlated with the timodal distribution of DMH-induced adenocarcinomas (ACs). A unimodal peak in ACF of all sizes occurred between the sites of two distal ALNs. Thus, the distribution of ACF at 1 or 5 weeks did not correlate with distribution of AC found at 24 weeks. Of the 2640 ACF observed at 1 or at 5 weeks, none were found in the proximal 25% of the colon where ACs eventually occurred. It was concluded that: (1) ALNs play a promotional role in AC formation; (2) the ACs which form in the proximal quarter of the colon seldom if ever form via an ACF precursor; and (3) the location, the number and the size of ACF observed early after DMH exposure did not correlate with the location or predict the incidence of ACs which eventually formed in the colon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bland P. W., Britton D. C. Morphological study of antigen-sampling structures in the rat large intestine. Infect Immun. 1984 Feb;43(2):693–699. doi: 10.1128/iai.43.2.693-699.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caderni G., Giannini A., Lancioni L., Luceri C., Biggeri A., Dolara P. Characterisation of aberrant crypt foci in carcinogen-treated rats: association with intestinal carcinogenesis. Br J Cancer. 1995 Apr;71(4):763–769. doi: 10.1038/bjc.1995.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. W., Lancaster H. K., Hardman W. E., Cameron I. L. Distribution of intestine-associated lymphoid tissue, aberrant crypt foci, and tumors in the large bowel of 1,2-dimethylhydrazine-treated mice. Cancer Res. 1994 Aug 15;54(16):4304–4307. [PubMed] [Google Scholar]
- Deschner E. E., Lytle J. S., Wong G., Ruperto J. F., Newmark H. L. The effect of dietary omega-3 fatty acids (fish oil) on azoxymethanol-induced focal areas of dysplasia and colon tumor incidence. Cancer. 1990 Dec 1;66(11):2350–2356. doi: 10.1002/1097-0142(19901201)66:11<2350::aid-cncr2820661117>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hardman W. E., Cameron I. L. Colonic crypts located over lymphoid nodules of 1,2-dimethylhydrazine-treated rats are hyperplastic and at high risk of forming adenocarcinomas. Carcinogenesis. 1994 Oct;15(10):2353–2361. doi: 10.1093/carcin/15.10.2353. [DOI] [PubMed] [Google Scholar]
- Hardman W. E., Cameron I. L., Heitman D. W., Contreras E. Demonstration of the need for end point validation of putative biomarkers: failure of aberrant crypt foci to predict colon cancer incidence. Cancer Res. 1991 Dec 1;51(23 Pt 1):6388–6392. [PubMed] [Google Scholar]
- Jen J., Powell S. M., Papadopoulos N., Smith K. J., Hamilton S. R., Vogelstein B., Kinzler K. W. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 1994 Nov 1;54(21):5523–5526. [PubMed] [Google Scholar]
- Lam L. K., Zhang J. Reduction of aberrant crypt formation in the colon of CF1 mice by potential chemopreventive agents. Carcinogenesis. 1991 Dec;12(12):2311–2315. doi: 10.1093/carcin/12.12.2311. [DOI] [PubMed] [Google Scholar]
- Magnuson B. A., Bird R. P. Reduction of aberrant crypt foci induced in rat colon with azoxymethane or methylnitrosourea by feeding cholic acid. Cancer Lett. 1993 Jan 15;68(1):15–23. doi: 10.1016/0304-3835(93)90214-t. [DOI] [PubMed] [Google Scholar]
- Magnuson B. A., Carr I., Bird R. P. Ability of aberrant crypt foci characteristics to predict colonic tumor incidence in rats fed cholic acid. Cancer Res. 1993 Oct 1;53(19):4499–4504. [PubMed] [Google Scholar]
- Martin M. S., Hammann A., Martin F. Gut-associated lymphoid tissue and 1,2-dimethylhydrazine intestinal tumors in the rat: an histological and immunoenzymatic study. Int J Cancer. 1986 Jul 15;38(1):75–80. doi: 10.1002/ijc.2910380113. [DOI] [PubMed] [Google Scholar]
- McLellan E. A., Bird R. P. Aberrant crypts: potential preneoplastic lesions in the murine colon. Cancer Res. 1988 Nov 1;48(21):6187–6192. [PubMed] [Google Scholar]
- Nauss K. M., Locniskar M., Pavlina T., Newberne P. M. Morphology and distribution of 1,2-dimethylhydrazine dihydrochloride-induced colon tumors and their relationship to gut-associated lymphoid tissue in the rat. J Natl Cancer Inst. 1984 Oct;73(4):915–924. [PubMed] [Google Scholar]
- Pereira M. A., Barnes L. H., Rassman V. L., Kelloff G. V., Steele V. E. Use of azoxymethane-induced foci of aberrant crypts in rat colon to identify potential cancer chemopreventive agents. Carcinogenesis. 1994 May;15(5):1049–1054. doi: 10.1093/carcin/15.5.1049. [DOI] [PubMed] [Google Scholar]
- Pereira M. A., Khoury M. D. Prevention by chemopreventive agents of azoxymethane-induced foci of aberrant crypts in rat colon. Cancer Lett. 1991 Dec 9;61(1):27–33. doi: 10.1016/0304-3835(91)90073-q. [DOI] [PubMed] [Google Scholar]
- Pretlow T. P. Aberrant crypt foci and K-ras mutations: earliest recognized players or innocent bystanders in colon carcinogenesis? Gastroenterology. 1995 Feb;108(2):600–603. doi: 10.1016/0016-5085(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A. M., Hogan M. L. Large intestinal carcinogenesis. II. Histogenesis and unusual features of low-dose azoxymethane-induced carcinomas in F344 rats. J Natl Cancer Inst. 1984 Dec;73(6):1297–1305. [PubMed] [Google Scholar]
- Shimamoto F., Vollmer E. Changes in intestinal mucosa above lymph follicles during carcinogenesis in rats. A light and electron microscopic study. J Cancer Res Clin Oncol. 1987;113(1):41–50. doi: 10.1007/BF00389965. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Stern H. S., Penner M., Hay K., Mitri A., Bapat B. V., Gallinger S. Somatic APC and K-ras codon 12 mutations in aberrant crypt foci from human colons. Cancer Res. 1994 Nov 1;54(21):5527–5530. [PubMed] [Google Scholar]
- Thorup I., Meyer O., Kristiansen E. Influence of a dietary fiber on development of dimethylhydrazine-induced aberrant crypt foci and colon tumor incidence in Wistar rats. Nutr Cancer. 1994;21(2):177–182. doi: 10.1080/01635589409514315. [DOI] [PubMed] [Google Scholar]
- Tudek B., Bird R. P., Bruce W. R. Foci of aberrant crypts in the colons of mice and rats exposed to carcinogens associated with foods. Cancer Res. 1989 Mar 1;49(5):1236–1240. [PubMed] [Google Scholar]
- Wargovich M. J., Harris C., Chen C. D., Palmer C., Steele V. E., Kelloff G. J. Growth kinetics and chemoprevention of aberrant crypts in the rat colon. J Cell Biochem Suppl. 1992;16G:51–54. doi: 10.1002/jcb.240501110. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Watanabe K. Quantitative kinetics of development of N-2-fluorenylacetamide-induced, altered (hyperplastic) hepatocellular foci resistant to iron accumulation and of their reversion or persistence following removal of carcinogen. J Natl Cancer Inst. 1978 Jul;61(1):113–121. doi: 10.1093/jnci/61.1.113. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Minamoto T., Ochiai A., Onda M., Esumi H. Frequent and characteristic K-ras activation and absence of p53 protein accumulation in aberrant crypt foci of the colon. Gastroenterology. 1995 Feb;108(2):434–440. doi: 10.1016/0016-5085(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Zhang X. M., Stamp D., Minkin S., Medline A., Corpet D. E., Bruce W. R., Archer M. C. Promotion of aberrant crypt foci and cancer in rat colon by thermolyzed protein. J Natl Cancer Inst. 1992 Jul 1;84(13):1026–1030. doi: 10.1093/jnci/84.13.1026. [DOI] [PubMed] [Google Scholar]



