Abstract
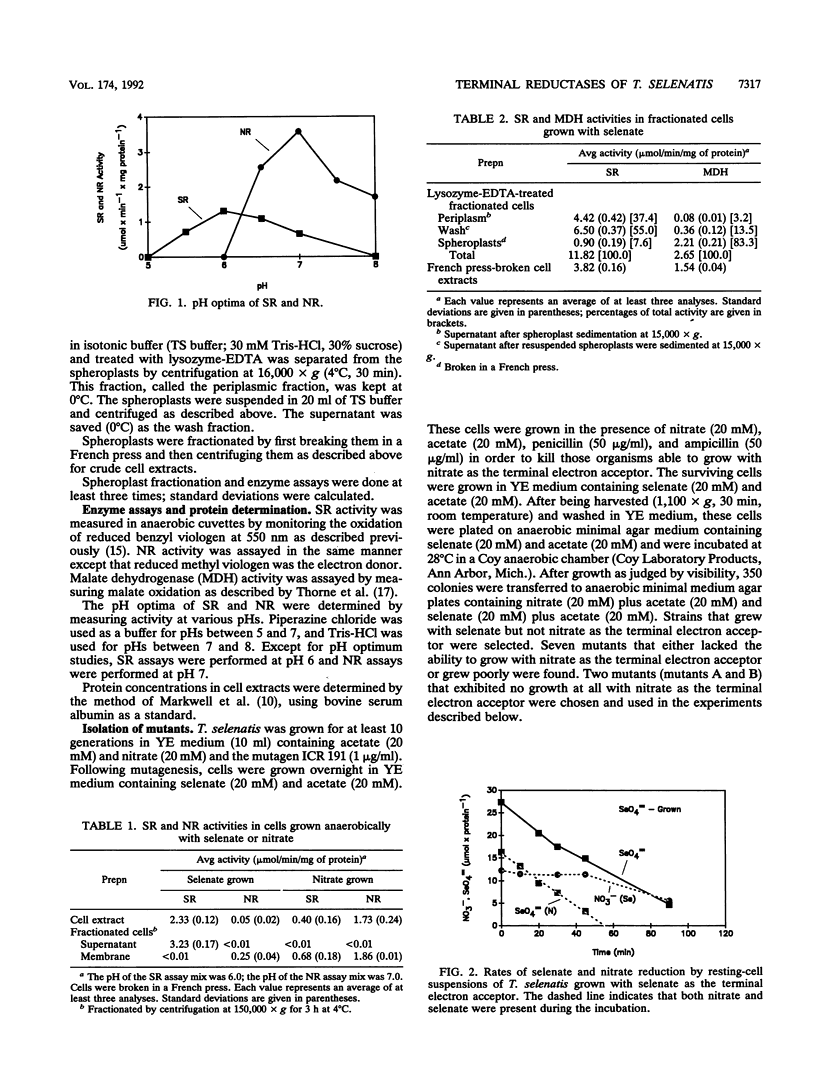
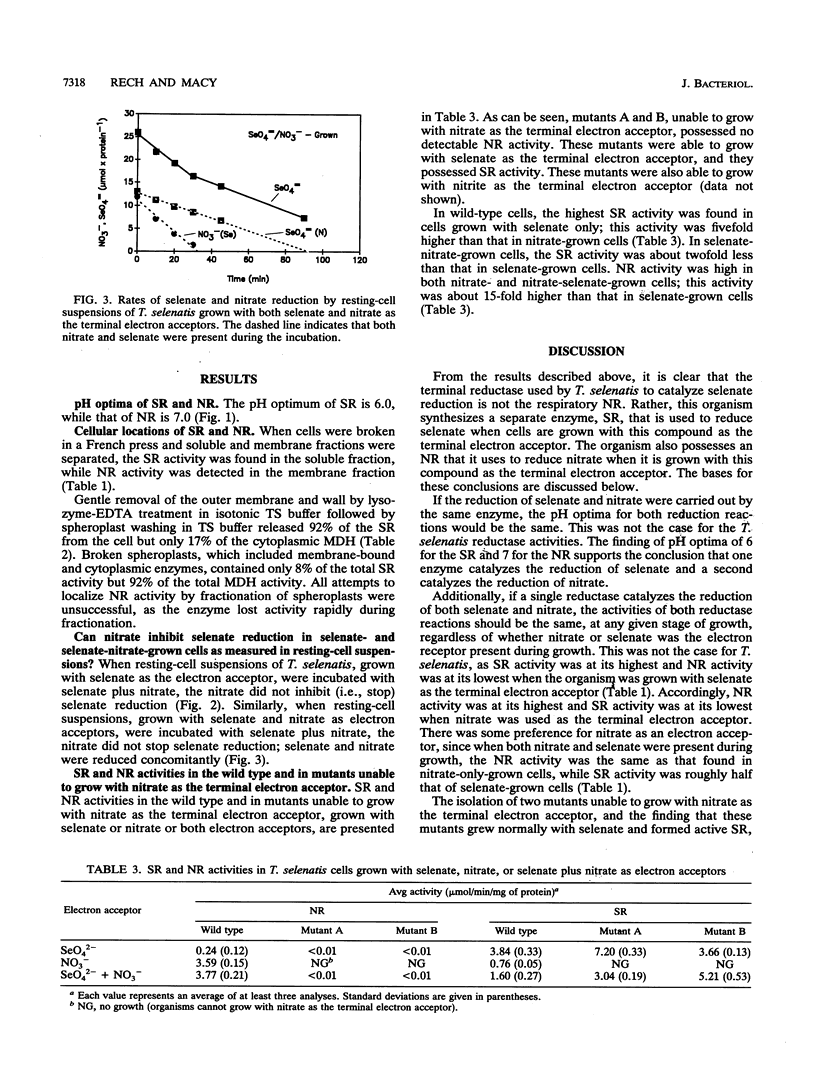
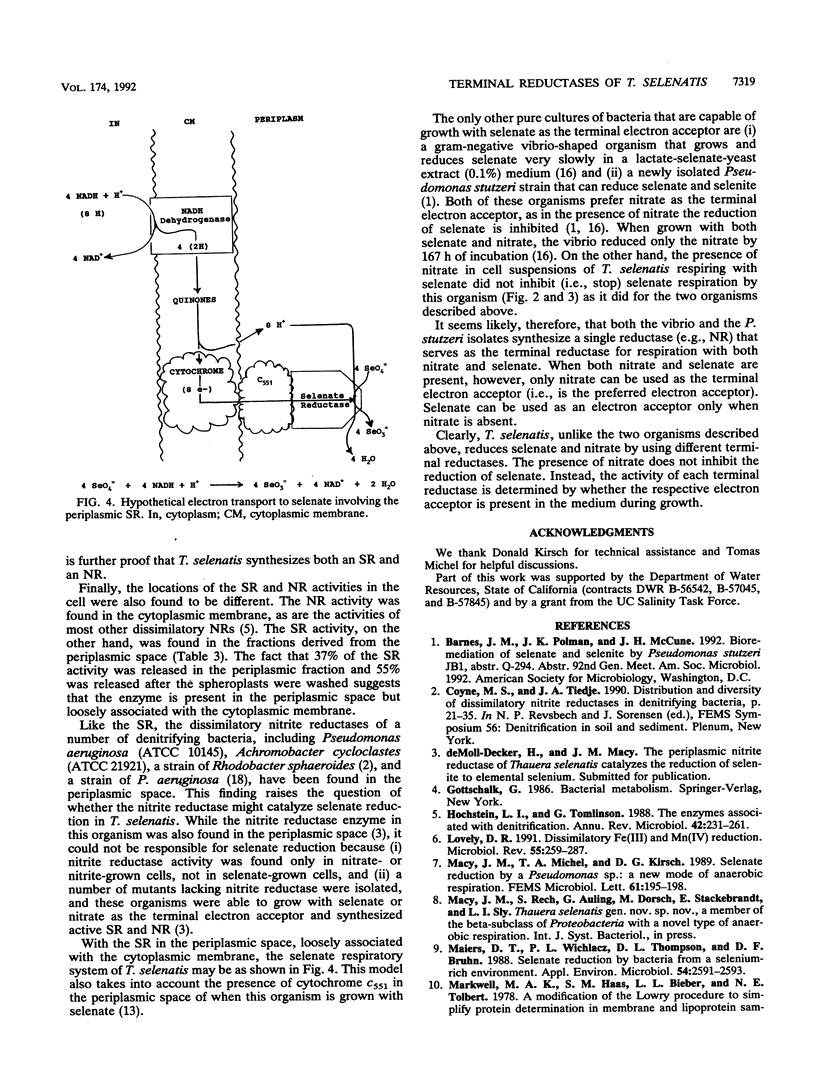
A number of approaches have been used to show that a recently isolated selenate-respiring bacterium, Thauera selenatis, is able to synthesize both a selenate reductase (SR) and a nitrate reductase (NR). (i) The pH optimum of the SR was found to be 6.0; that of the NR was 7.0. (ii) The presence of nitrate did not inhibit selenate reduction in selenate-grown cells. (iii) In cell extracts, the highest SR or NR activity was observed in cells grown with the respective electron acceptor. (iv) Mutants that were unable to grow with nitrate as the terminal electron acceptor and lacked NR activity were isolated; these mutants grew normally with selenate and synthesized SR. (v) The SR was found in the periplasmic space of the cell, whereas the NR was present in the cytoplasmic membrane. A hypothetical electron transport system involving the SR is described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hochstein L. I., Tomlinson G. A. The enzymes associated with denitrification. Annu Rev Microbiol. 1988;42:231–261. doi: 10.1146/annurev.mi.42.100188.001311. [DOI] [PubMed] [Google Scholar]
- Lovley D. R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991 Jun;55(2):259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J. M., Michel T. A., Kirsch D. G. Selenate reduction by a Pseudomonas species: a new mode of anaerobic respiration. FEMS Microbiol Lett. 1989 Oct 1;52(1-2):195–198. doi: 10.1016/0378-1097(89)90195-x. [DOI] [PubMed] [Google Scholar]
- Maiers D. T., Wichlacz P. L., Thompson D. L., Bruhn D. F. Selenate reduction by bacteria from a selenium-rich environment. Appl Environ Microbiol. 1988 Oct;54(10):2591–2593. doi: 10.1128/aem.54.10.2591-2593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Melville S. B., Michel T. A., Macy J. M. Regulation of carbon flow in Selenomonas ruminantium grown in glucose-limited continuous culture. J Bacteriol. 1988 Nov;170(11):5305–5311. doi: 10.1128/jb.170.11.5305-5311.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Steinberg N. A., Blum J. S., Hochstein L., Oremland R. S. Nitrate is a preferred electron acceptor for growth of freshwater selenate-respiring bacteria. Appl Environ Microbiol. 1992 Jan;58(1):426–428. doi: 10.1128/aem.58.1.426-428.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. J., GROSSMAN L. I., KAPLAN N. O. Starch-gel electrophoresis of malate dehydrogenase. Biochim Biophys Acta. 1963 Jun 11;73:193–203. doi: 10.1016/0006-3002(63)90303-2. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Periplasmic location of the terminal reductase in nitrite respiration. FEBS Lett. 1978 Aug 15;92(2):214–218. doi: 10.1016/0014-5793(78)80757-1. [DOI] [PubMed] [Google Scholar]


