Abstract
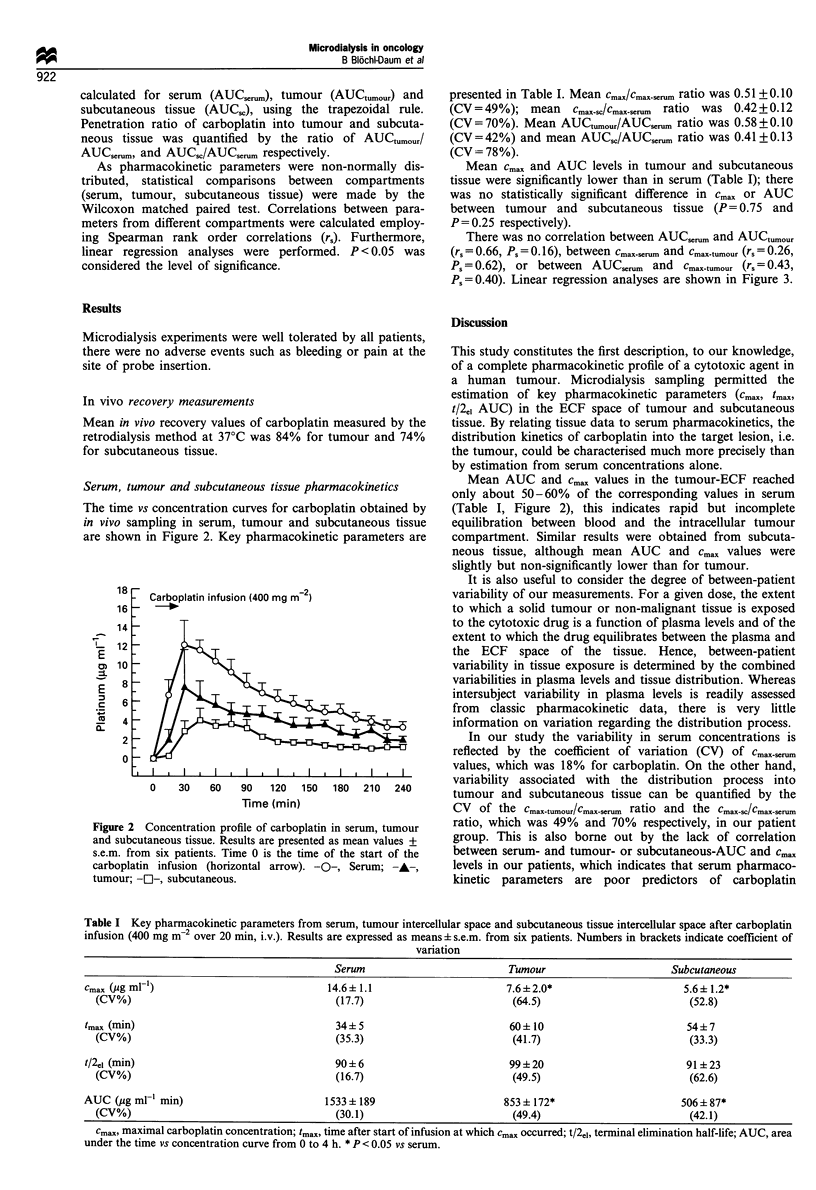
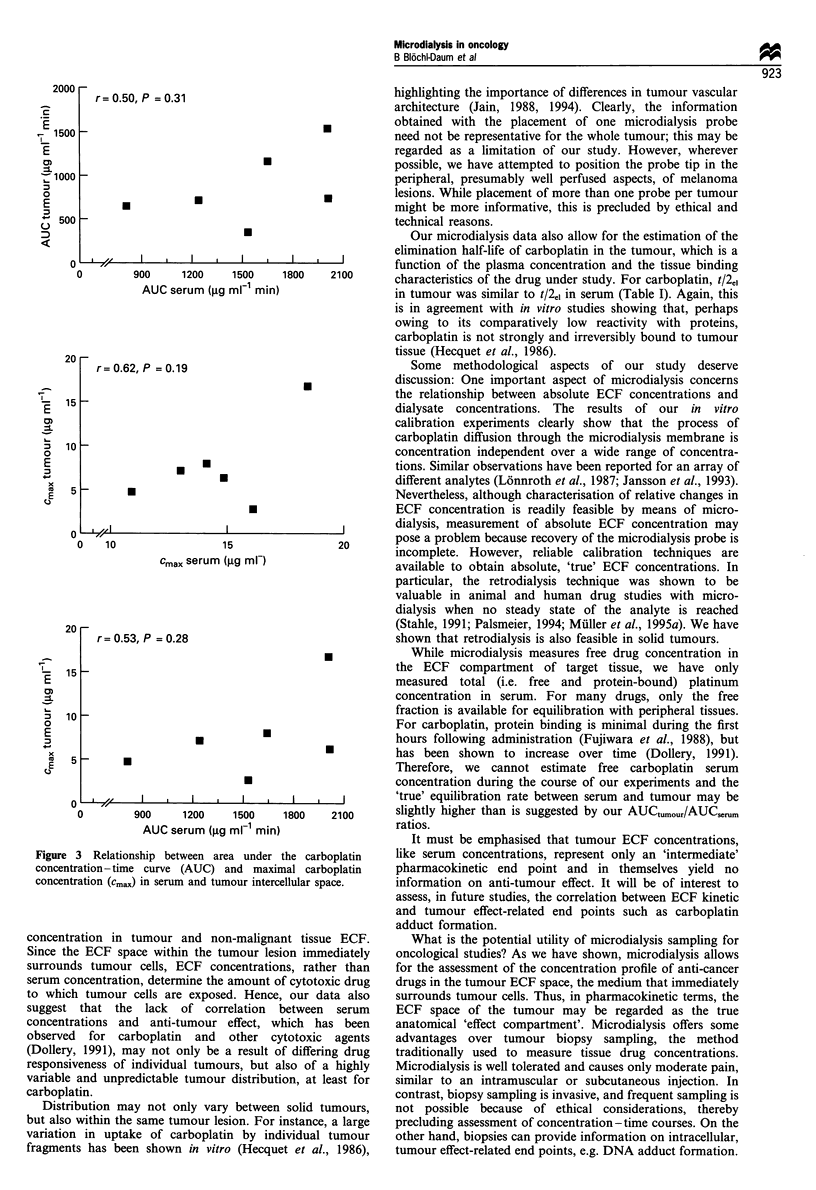
Clinical anti-tumour efficacy of anti-cancer drugs is a function of dose intensity, i.e. the concentration--time profile in tumour tissue. Hence, information on drug concentration profiles in tumours is of critical importance but appropriate methods for measurement are lacking. The aim of the present study was to obtain, by microdialysis sampling, concentration--time profiles in a solid tumour (melanoma) of a model anti-cancer drug, carboplatin, and thereby to assess the scope of microdialysis for tumour pharmacokinetic studies in man. Six patients with cutaneous melanoma metastases at the extremities or body trunk, scheduled to receive carboplatin (400 mg m-2 i.v.) were studied. Carboplatin concentrations were monitored in serum, intratumoral and subcutaneous tissue. Calibration of the microdialysis probes was carried out in vitro and in vivo with use of the retrodialysis method. Complete carboplatin concentration vs time profiles in tumour and subcutaneous tissue were obtained. Major pharmacokinetic parameters (maximum concentration, time to maximum concentration, area under the curve, elimination half-life) were calculated for tissues and tumour/serum concentration ratios for carboplatin were derived. Mean free concentrations of carboplatin in cutaneous melanoma metastases reached only about 50-60% of total serum levels; maximal intratumoral concentrations were 7.6 (+/-2.0; s.e.m.) microgram/ml, mean concentrations in subcutaneous tissue were similar to those in tumour. The present study demonstrates that microdialysis is a novel tool for measuring drug concentrations in solid tumours in humans in vivo and appears to be a valuable addition for pharmacokinetic/pharmacodynamic studies in oncology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolinder J., Ungerstedt U., Arner P. Long-term continuous glucose monitoring with microdialysis in ambulatory insulin-dependent diabetic patients. Lancet. 1993 Oct 30;342(8879):1080–1085. doi: 10.1016/0140-6736(93)92063-y. [DOI] [PubMed] [Google Scholar]
- Carneheim C., Ståhle L. Microdialysis of lipophilic compounds: a methodological study. Pharmacol Toxicol. 1991 Nov;69(5):378–380. doi: 10.1111/j.1600-0773.1991.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Coughlin C. T., Richmond R. C., Page R. L. Platinum drug delivery and radiation for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 1994 Mar 1;28(4):1029–1038. doi: 10.1016/0360-3016(94)90125-2. [DOI] [PubMed] [Google Scholar]
- DeConti R. C., Toftness B. R., Lange R. C., Creasey W. A. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Res. 1973 Jun;33(6):1310–1315. [PubMed] [Google Scholar]
- Eskey C. J., Koretsky A. P., Domach M. M., Jain R. K. 2H-nuclear magnetic resonance imaging of tumor blood flow: spatial and temporal heterogeneity in a tissue-isolated mammary adenocarcinoma. Cancer Res. 1992 Nov 1;52(21):6010–6019. [PubMed] [Google Scholar]
- Fujiwara K., Miyagi Y., Hayase R., Yoshinouchi M., Kobashi Y., Kohno I., Sekiba K. [Pharmacokinetics of carboplatin (CBDCA) and tissue concentration of platinum in gynecologic organs]. Gan To Kagaku Ryoho. 1988 Jun;15(6):1943–1948. [PubMed] [Google Scholar]
- Hecquet B., Leroy A., Lefebvre J. L., Peyrat J. P., Adenis L. Uptake of platinum compounds in human tumors. In vitro study. Bull Cancer. 1986;73(5):535–541. [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Jansson P. A., Fowelin J. P., von Schenck H. P., Smith U. P., Lönnroth P. N. Measurement by microdialysis of the insulin concentration in subcutaneous interstitial fluid. Importance of the endothelial barrier for insulin. Diabetes. 1993 Oct;42(10):1469–1473. doi: 10.2337/diab.42.10.1469. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Carlsten J., Johnson L., Smith U. Measurements by microdialysis of free tissue concentrations of propranolol. J Chromatogr. 1991 Aug 23;568(2):419–425. doi: 10.1016/0378-4347(91)80179-g. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987 Aug;253(2 Pt 1):E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Morrison P. F., Bungay P. M., Hsiao J. K., Ball B. A., Mefford I. N., Dedrick R. L. Quantitative microdialysis: analysis of transients and application to pharmacokinetics in brain. J Neurochem. 1991 Jul;57(1):103–119. doi: 10.1111/j.1471-4159.1991.tb02105.x. [DOI] [PubMed] [Google Scholar]
- Müller M., Schmid R., Georgopoulos A., Buxbaum A., Wasicek C., Eichler H. G. Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther. 1995 Apr;57(4):371–380. doi: 10.1016/0009-9236(95)90205-8. [DOI] [PubMed] [Google Scholar]
- Palsmeier R. K., Lunte C. E. Microdialysis sampling in tumor and muscle: study of the disposition of 3-amino-1,2,4-benzotriazine-1,4-di-N-oxide (SR 4233). Life Sci. 1994;55(10):815–825. doi: 10.1016/0024-3205(94)00565-6. [DOI] [PubMed] [Google Scholar]
- Pich E. M., Koob G. F., Heilig M., Menzaghi F., Vale W., Weiss F. Corticotropin-releasing factor release from the mediobasal hypothalamus of the rat as measured by microdialysis. Neuroscience. 1993 Aug;55(3):695–707. doi: 10.1016/0306-4522(93)90435-i. [DOI] [PubMed] [Google Scholar]
- SKIPPER H. E., SCHABEL F. M., Jr, WILCOX W. S. EXPERIMENTAL EVALUATION OF POTENTIAL ANTICANCER AGENTS. XIV. FURTHER STUDY OF CERTAIN BASIC CONCEPTS UNDERLYING CHEMOTHERAPY OF LEUKEMIA. Cancer Chemother Rep. 1965 Apr;45:5–28. [PubMed] [Google Scholar]
- Scheyer R. D., During M. J., Spencer D. D., Cramer J. A., Mattson R. H. Measurement of carbamazepine and carbamazepine epoxide in the human brain using in vivo microdialysis. Neurology. 1994 Aug;44(8):1469–1472. doi: 10.1212/wnl.44.8.1469. [DOI] [PubMed] [Google Scholar]
- Ståhle L., Arner P., Ungerstedt U. Drug distribution studies with microdialysis. III: Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49(24):1853–1858. doi: 10.1016/0024-3205(91)90488-w. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Microdialysis--principles and applications for studies in animals and man. J Intern Med. 1991 Oct;230(4):365–373. doi: 10.1111/j.1365-2796.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Vaden S. L., Williams P. L., Page R. L., Riviere J. E. Effect of tumor presence on cisplatin and carboplatin: disposition in the isolated, perfused tumor and skin flap. Cancer Chemother Pharmacol. 1993;32(1):31–38. doi: 10.1007/BF00685873. [DOI] [PubMed] [Google Scholar]