Abstract
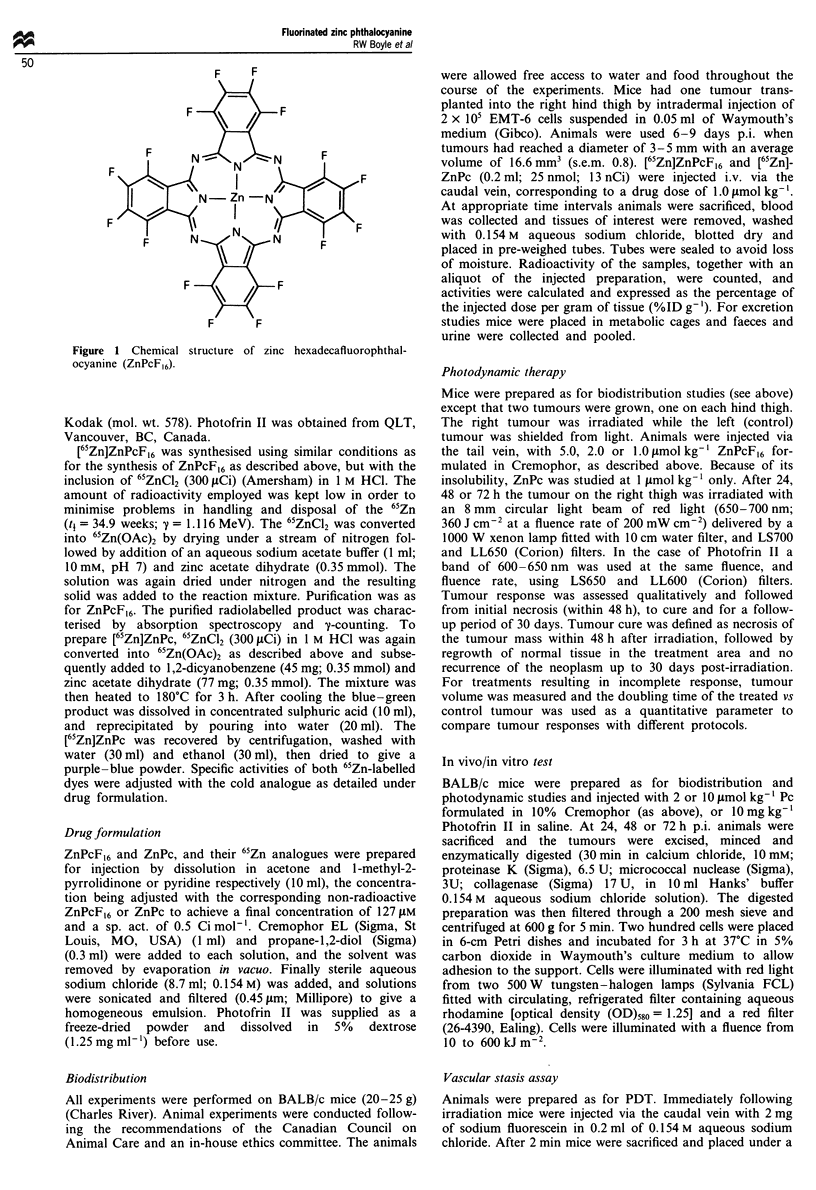
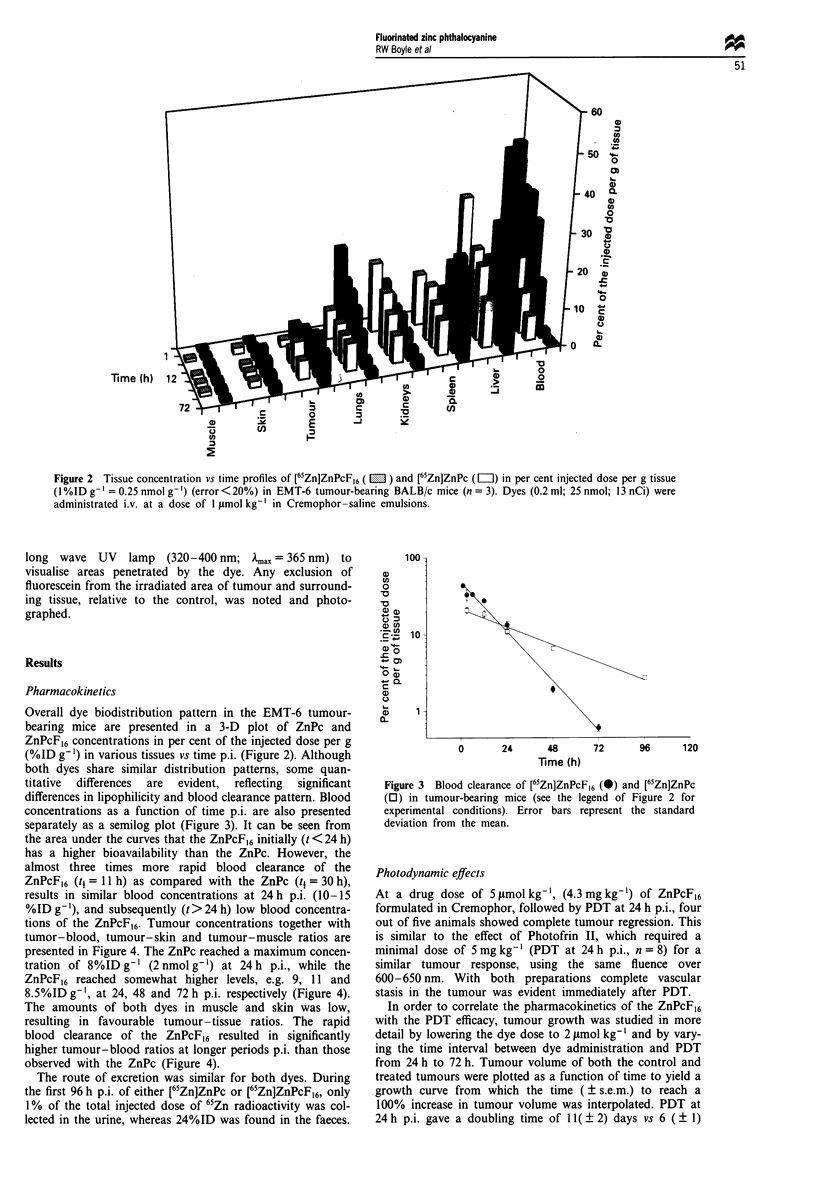
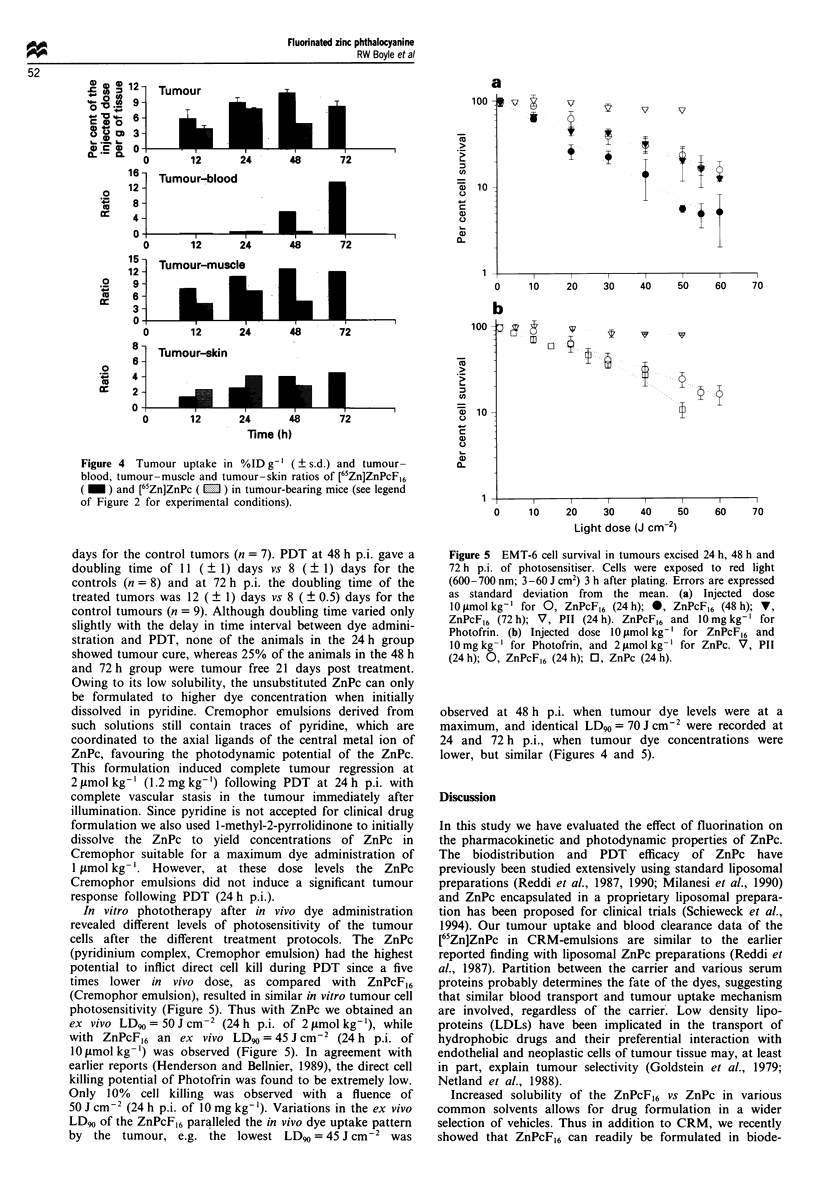
Hexadecafluorinated zinc phthalocyanine (ZnPcF16), an analogue of zinc phthalocyanine (ZnPc) in which all hydrogen atoms have been substituted by fluorine, was prepared as a single isomeric product via the condensation of tetrafluorophthalonitrile with zinc acetate. Fluorination renders the ZnPc soluble in most common solvents. The photodynamic properties and pharmacokinetics of the ZnPcF16 were evaluated in EMT-6 tumour-bearing Balb/c mice using 65Zn-radiolabelled analogues. Both dyes, administered i.v. at 1 mumol kg-1 as Cremophor emulsions, revealed good tumour uptake [approximately 8-9 per cent of the injected dose per g tissue (%IDg-1)] at 24 h post injection (p.i.), with the fluorinated dye reaching higher concentrations (approximately 11%IDg-1) at 48 h p.i. and subsequently higher tumour-blood ratios due to rapid blood clearance. ZnPcF16 at a dose of 5 mumol kg-1 (4.3 mg kg-1) induced complete tumour regression after phototherapy (24 h p.i., 650-700 nm band, 360 J cm-2, 200 mW cm-1). At a dose of 2 mumol kg-1 and phototherapy at 24 h p.i., the tumour volume doubling time increased to 11 days vs 6 days for the control tumours. A similar tumour growth delay was observed when phototherapy was conducted at 48 h or 72 h after dye injection implying that tumour response correlates with tumour dye concentrations rather than serum concentrations. As a result of its low solubility, the administered dose of ZnPc was limited to 1 mumol kg-1 and at this drug level significant tumour response was only observed when the dye was solubilised as the pyridinium salt. Isolation of the neoplastic cells after in vivo dye administration and in vitro exposure to red light followed by a colony formation assay showed that the ZnPcF16 exhibited a 1-2 order of magnitude higher potential for direct cell killing as compared with Photofrin and about a five times lower efficiency than ZnPc. However, all three photosensitisers induced complete occlusion of tumour vasculature immediately after PDT, suggesting that tumour regression mainly resulted from vascular stasis. The ZnPcF16 offers several advantages over ZnPc for clinical applications, including improved solubility in most solvents, resulting in facilitated drug formation, favourable pharmacokinetics as well as the potential use in fluorine magnetic resonance (F-MR) imaging.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allémann E., Brasseur N., Benrezzak O., Rousseau J., Kudrevich S. V., Boyle R. W., Leroux J. C., Gurny R., Van Lier J. E. PEG-coated poly(lactic acid) nanoparticles for the delivery of hexadecafluoro zinc phthalocyanine to EMT-6 mouse mammary tumours. J Pharm Pharmacol. 1995 May;47(5):382–387. doi: 10.1111/j.2042-7158.1995.tb05815.x. [DOI] [PubMed] [Google Scholar]
- Ginevra F., Biffanti S., Pagnan A., Biolo R., Reddi E., Jori G. Delivery of the tumour photosensitizer zinc(II)-phthalocyanine to serum proteins by different liposomes: studies in vitro and in vivo. Cancer Lett. 1990 Jan;49(1):59–65. doi: 10.1016/0304-3835(90)90139-o. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Bellnier D. A. Tissue localization of photosensitizers and the mechanism of photodynamic tissue destruction. Ciba Found Symp. 1989;146:112–130. doi: 10.1002/9780470513842.ch8. [DOI] [PubMed] [Google Scholar]
- Langlois R., Ali H., Brasseur N., Wagner J. R., van Lier J. E. Biological activities of phythalocyanines--IV. Type II sensitized photooxidation of L-tryptophan and cholesterol by sulfonated metallo phthalocyanines. Photochem Photobiol. 1986 Aug 2;44(2):117–123. doi: 10.1111/j.1751-1097.1986.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Milanesi C., Zhou C., Biolo R., Jori G. Zn(II)-phthalocyanine as a photodynamic agent for tumours. II. Studies on the mechanism of photosensitised tumour necrosis. Br J Cancer. 1990 Jun;61(6):846–850. doi: 10.1038/bjc.1990.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland P. A., Zetter B. R., Via D. P., Voyta J. C. In situ labelling of vascular endothelium with fluorescent acetylated low density lipoprotein. Histochem J. 1985 Dec;17(12):1309–1320. doi: 10.1007/BF01002528. [DOI] [PubMed] [Google Scholar]
- Reddi E., Lo Castro G., Biolo R., Jori G. Pharmacokinetic studies with zinc(II)-phthalocyanine in tumour-bearing mice. Br J Cancer. 1987 Nov;56(5):597–600. doi: 10.1038/bjc.1987.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi E., Zhou C., Biolo R., Menegaldo E., Jori G. Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency. Br J Cancer. 1990 Mar;61(3):407–411. doi: 10.1038/bjc.1990.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal I. Phthalocyanines as photodynamic sensitizers. Photochem Photobiol. 1991 Jun;53(6):859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Zalar G. L., Poh-Fitzpatrick M., Krohn D. L., Jacobs R., Harber L. C. Induction of drug photosensitization in man after parenteral exposure to hematoporphyrin. Arch Dermatol. 1977 Oct;113(10):1392–1397. [PubMed] [Google Scholar]


