Abstract
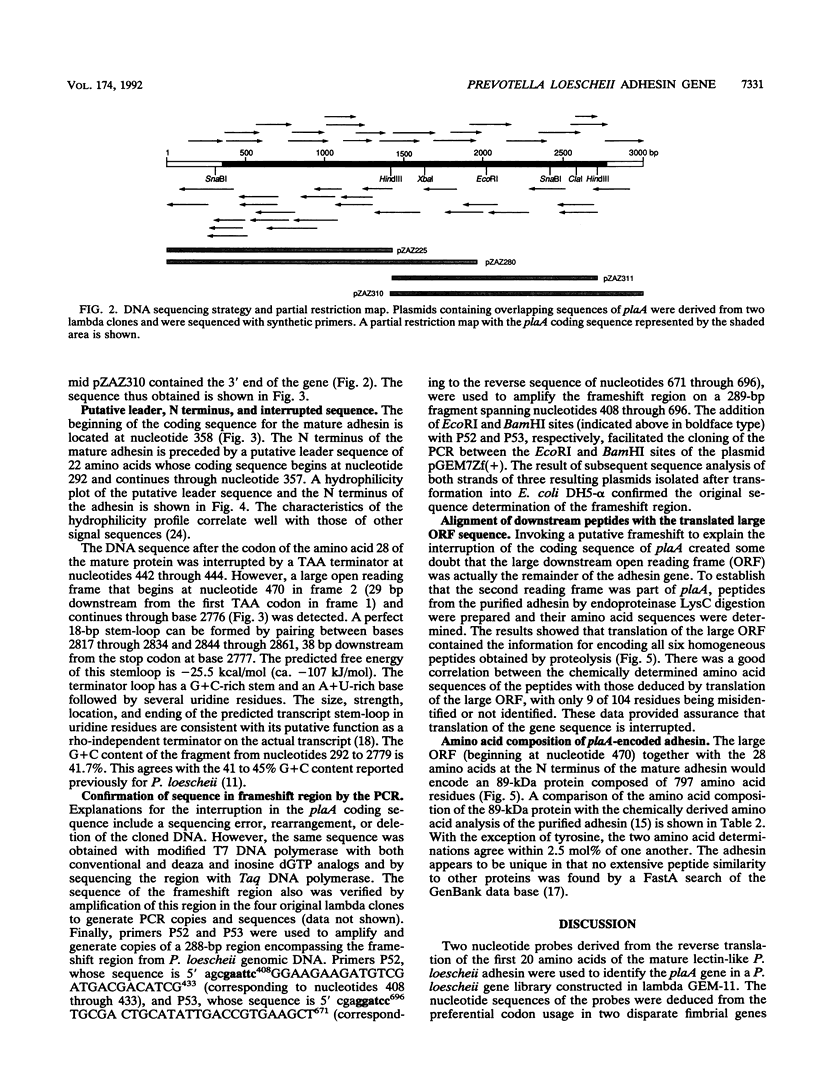
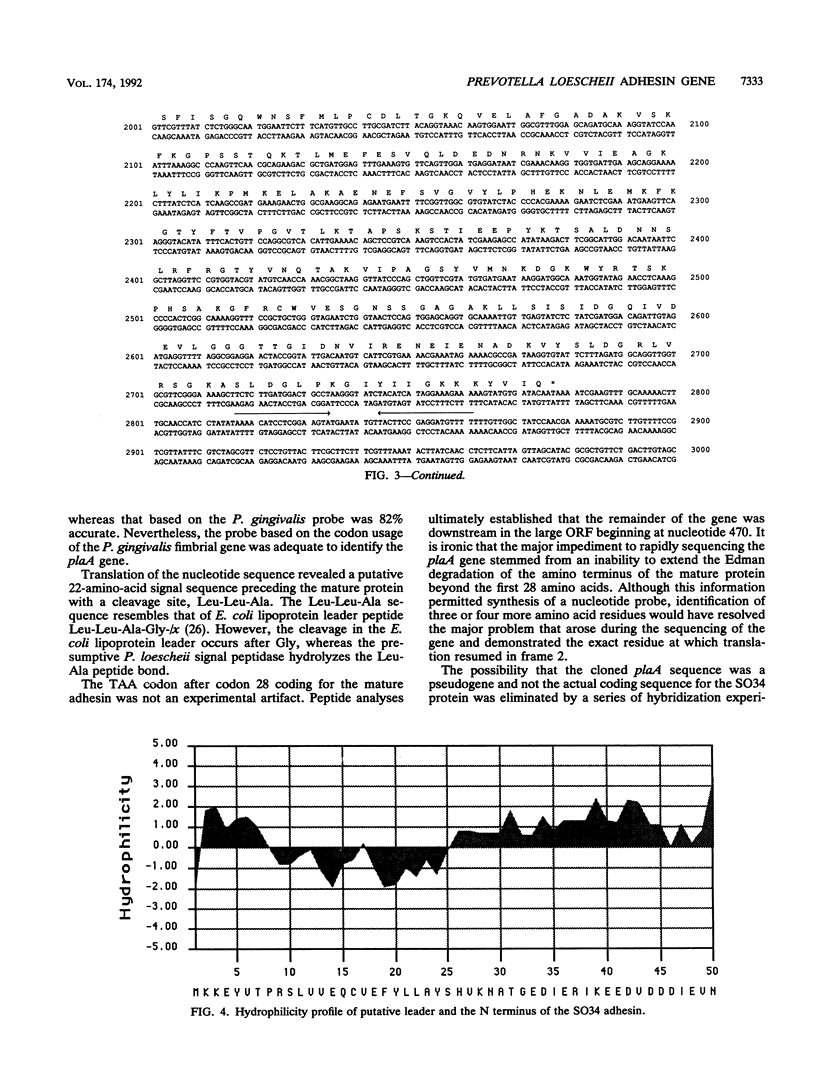
We cloned and sequenced the Prevotella loescheii gene plaA, which encodes a lectin-like adhesin that mediates the coaggregation of P. loescheii 1295 with Streptococcus oralis 34. A probe derived from the N-terminal amino acid sequence of the purified adhesin was used to identify the plaA gene from a P. loescheii genomic library constructed in lambda GEM-11. Sequence analysis of plaA indicates that the initial translation product contains a 22-amino-acid leader. The reading frame of the plaA gene is interrupted after amino acid 28 of the mature protein by a TAA termination codon. Amplification of the P. loescheii genomic DNA in the region surrounding this codon by the polymerase chain reaction followed by DNA sequencing of the cloned DNA fragment established that this stop codon was not an experimental artifact. A frameshift beginning 29 bp downstream of the ochre terminator was required to access the only large open reading frame in the gene. Amino acid sequences of six purified peptides derived by limited proteolysis of adhesin with endoproteinase Lys-C matched the downstream amino acid sequence derived by translation of the large open reading frame. The gene coding sequence of 2.4 kb contains sufficient information for the synthesis of an 89-kDa protein. A putative rho-independent terminator (delta G = -25.5 kcal/mol [ca. -107 kJ/mol]) was detected 38 bp downstream from the plaA stop codon.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Weiss R. B., Gesteland R. F. Ribosome gymnastics--degree of difficulty 9.5, style 10.0. Cell. 1990 Aug 10;62(3):413–423. doi: 10.1016/0092-8674(90)90007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A. Nucleotide sequence of the gene encoding pilin of Bacteroides nodosus, the causal organism of ovine footrot. J Bacteriol. 1984 Dec;160(3):1184–1187. doi: 10.1128/jb.160.3.1184-1187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Murray N., Lehrach H. Lambda phage vectors--EMBL series. Methods Enzymol. 1987;153:103–115. doi: 10.1016/0076-6879(87)53051-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E. Surface recognition among oral bacteria: multigeneric coaggregations and their mediators. Crit Rev Microbiol. 1989;17(2):137–159. doi: 10.3109/10408418909105746. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- London J., Allen J. Purification and characterization of a Bacteroides loeschei adhesin that interacts with procaryotic and eucaryotic cells. J Bacteriol. 1990 May;172(5):2527–2534. doi: 10.1128/jb.172.5.2527-2534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita O., Russell J. B., Wilson D. B. A Bacteroides ruminicola 1,4-beta-D-endoglucanase is encoded in two reading frames. J Bacteriol. 1991 Nov;173(21):6919–6926. doi: 10.1128/jb.173.21.6919-6926.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tempst P., Riviere L. Examination of automated polypeptide sequencing using standard phenyl isothiocyanate reagent and subpicomole high-performance liquid chromatographic analysis. Anal Biochem. 1989 Dec;183(2):290–300. doi: 10.1016/0003-2697(89)90482-x. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., Kolenbrander P. E., London J., Hand A. R., Andersen R. N. Fimbria-associated proteins of Bacteroides loescheii PK1295 mediate intergeneric coaggregations. J Bacteriol. 1987 Sep;169(9):4215–4222. doi: 10.1128/jb.169.9.4215-4222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Yu F., Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988 May 6;53(3):423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]



