Abstract
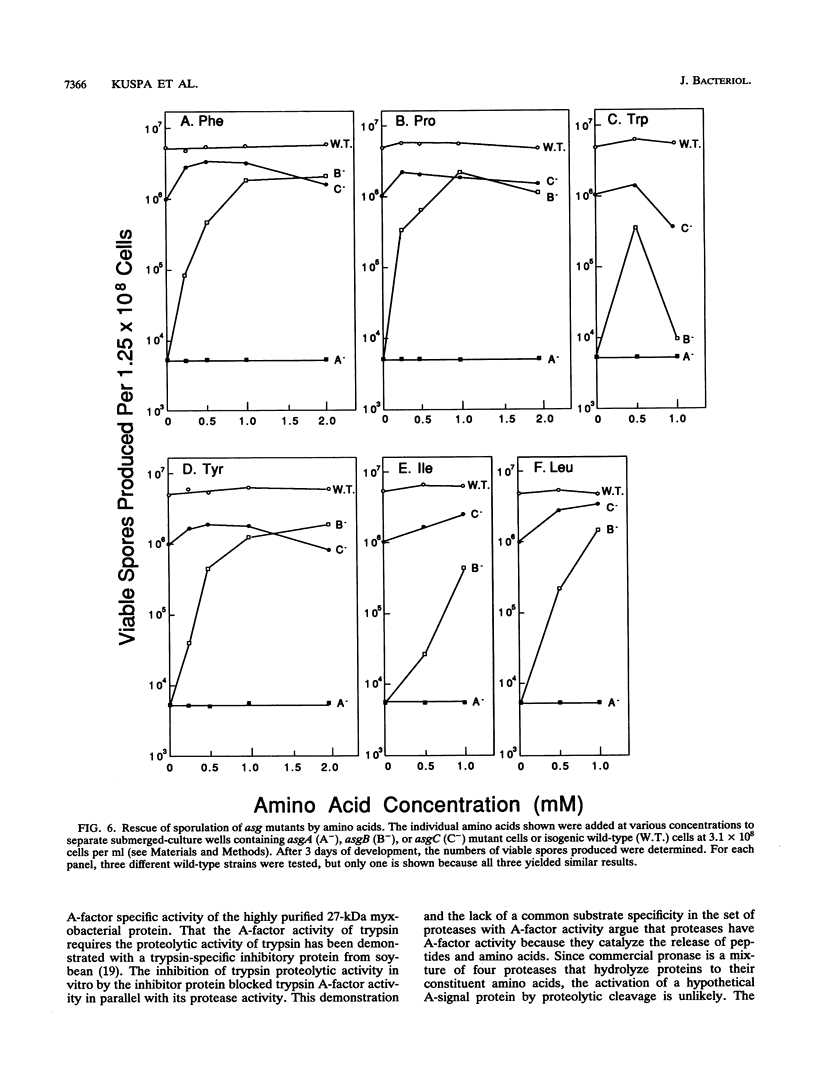
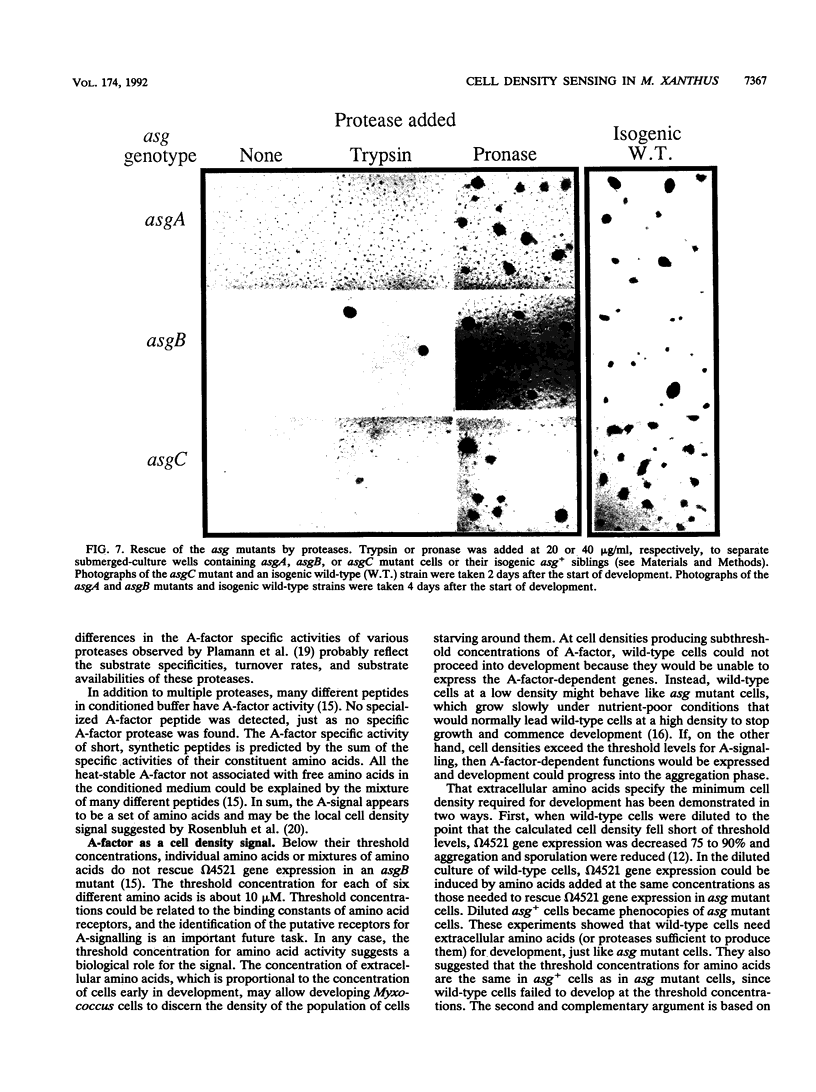
Mutations in any of three asg (A-signalling) loci cause fruiting body development of Myxococcus xanthus to arrest at about the 2-h stage. Development can be restored to asg mutants by the addition of conditioned buffer in which wild-type cells have been developing or of A-factor purified from the conditioned buffer. Two forms of A-factor have been identified: heat-stable A-factor, which is composed of amino acids and peptides, and heat-labile A-factor, which consists of at least two proteases. A-factor is found in conditioned buffer in rough proportion to the cell density. As decreasing amounts of either form of A-factor are added, the developmental response of asg cells decreases until a threshold concentration is reached, below which no response is detected. In addition, wild-type cells fail to develop when their density is decreased below the point at which the level of A-factor is predicted to fall short of this threshold. The development of low-density asg+ cells can, however, be restored by the addition of either form of A-factor. These experiments show that A-factor is important for the development of wild-type cells. Moreover, the development of an asgB mutant that produces 5 to 10% the wild-type level of A-factor can be restored when the cell density is increased 10-fold above the standard density. We propose that the A-signal is used by M. xanthus to specify the minimum cell density required for the initiation of development. Differences in the response to A-factor between different asg mutants suggest that the different asg loci govern A-factor production in diverse ways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. NUTRITIONAL REGU.ATION OF MORPHOGENESIS IN MYXOCOCCUS XANTHUS. J Bacteriol. 1963 Jul;86:67–72. doi: 10.1128/jb.86.1.67-72.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. Biology of the myxobacteria. Annu Rev Microbiol. 1966;20:75–106. doi: 10.1146/annurev.mi.20.100166.000451. [DOI] [PubMed] [Google Scholar]
- Kaplan H. B., Kuspa A., Kaiser D. Suppressors that permit A-signal-independent developmental gene expression in Myxococcus xanthus. J Bacteriol. 1991 Feb;173(4):1460–1470. doi: 10.1128/jb.173.4.1460-1470.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kuspa A., Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986 Sep;117(1):252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982 Jul;151(1):458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuspa A., Kaiser D. Genes required for developmental signalling in Myxococcus xanthus: three asg loci. J Bacteriol. 1989 May;171(5):2762–2772. doi: 10.1128/jb.171.5.2762-2772.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
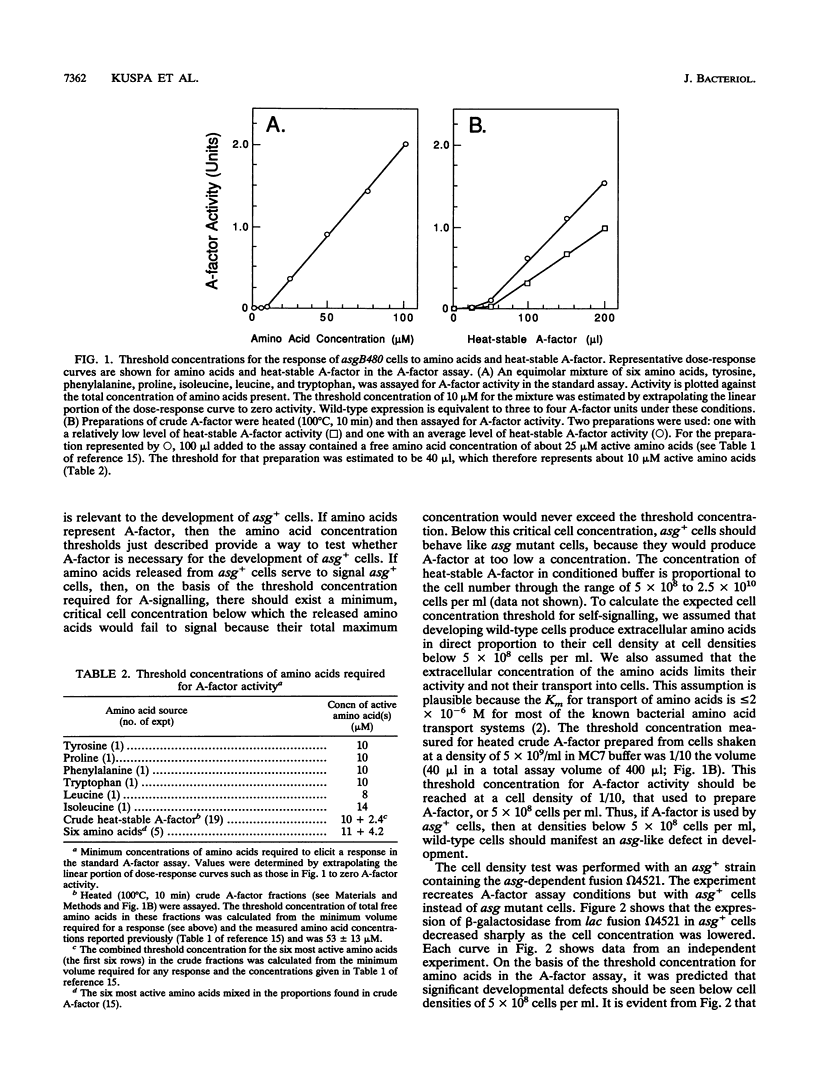
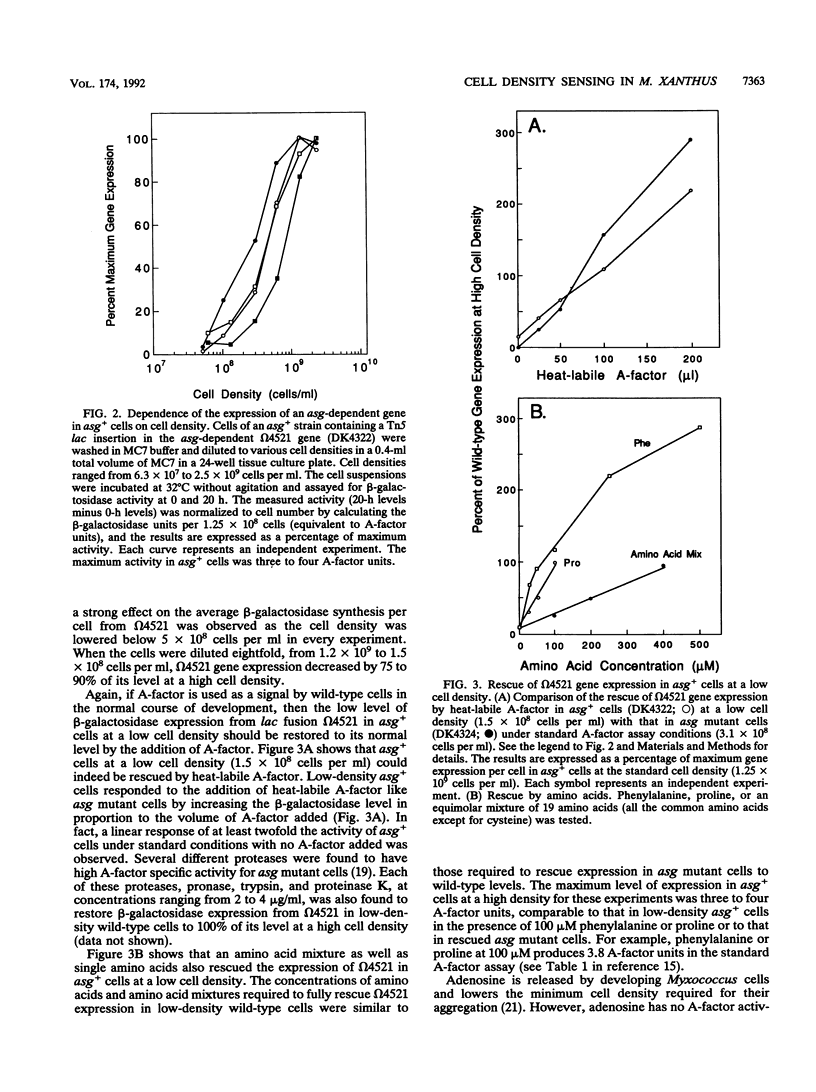
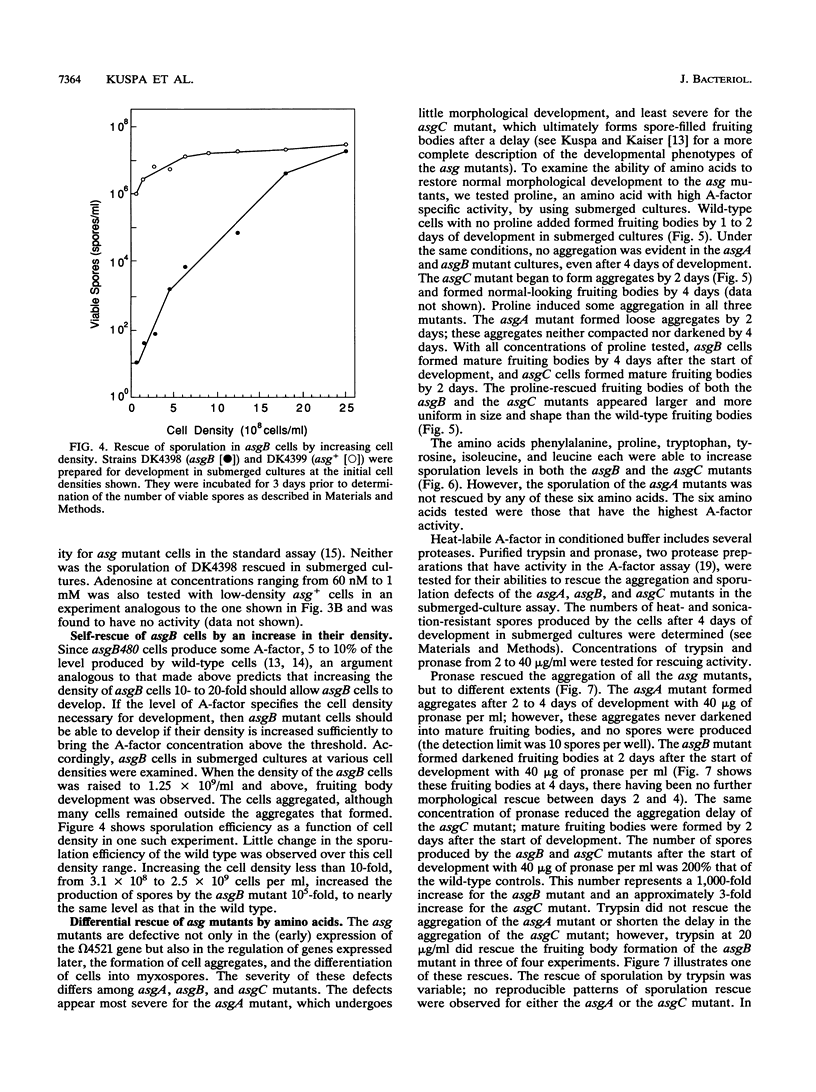
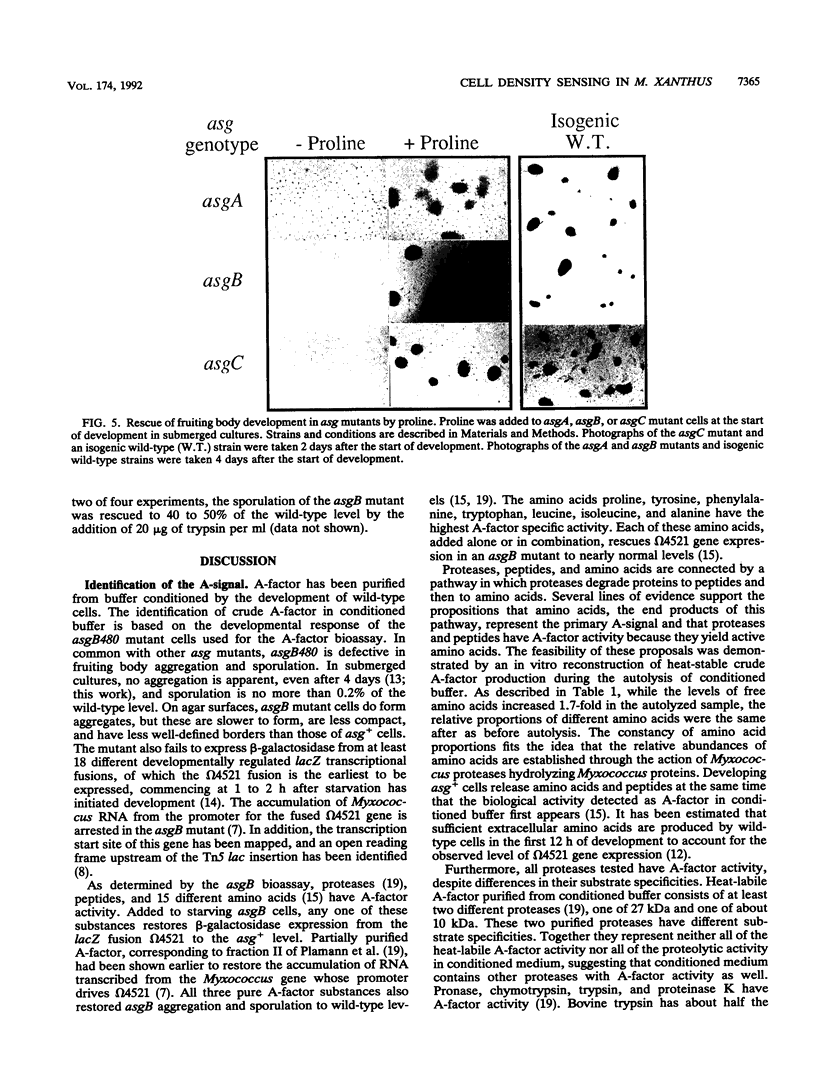
- Kuspa A., Kroos L., Kaiser D. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev Biol. 1986 Sep;117(1):267–276. doi: 10.1016/0012-1606(86)90369-6. [DOI] [PubMed] [Google Scholar]
- Kuspa A., Plamann L., Kaiser D. Identification of heat-stable A-factor from Myxococcus xanthus. J Bacteriol. 1992 May;174(10):3319–3326. doi: 10.1128/jb.174.10.3319-3326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRossa R., Kuner J., Hagen D., Manoil C., Kaiser D. Developmental cell interactions of Myxococcus xanthus: analysis of mutants. J Bacteriol. 1983 Mar;153(3):1394–1404. doi: 10.1128/jb.153.3.1394-1404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo K. A., Kaiser D. asgB, a gene required early for developmental signalling, aggregation, and sporulation of Myxococcus xanthus. Mol Gen Genet. 1989 Sep;218(3):409–418. doi: 10.1007/BF00332403. [DOI] [PubMed] [Google Scholar]
- Plamann L., Kuspa A., Kaiser D. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J Bacteriol. 1992 May;174(10):3311–3318. doi: 10.1128/jb.174.10.3311-3318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh A., Nir R., Sahar E., Rosenberg E. Cell-density-dependent lysis and sporulation of Myxococcus xanthus in agarose microbeads. J Bacteriol. 1989 Sep;171(9):4923–4929. doi: 10.1128/jb.171.9.4923-4929.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Dworkin M. Excreted adenosine is a cell density signal for the initiation of fruiting body formation in Myxococcus xanthus. Dev Biol. 1981 May;84(1):51–60. doi: 10.1016/0012-1606(81)90369-9. [DOI] [PubMed] [Google Scholar]
- Wireman J. W., Dworkin M. Morphogenesis and developmental interactions in myxobacteria. Science. 1975 Aug 15;189(4202):516–523. doi: 10.1126/science.806967. [DOI] [PubMed] [Google Scholar]





