Abstract
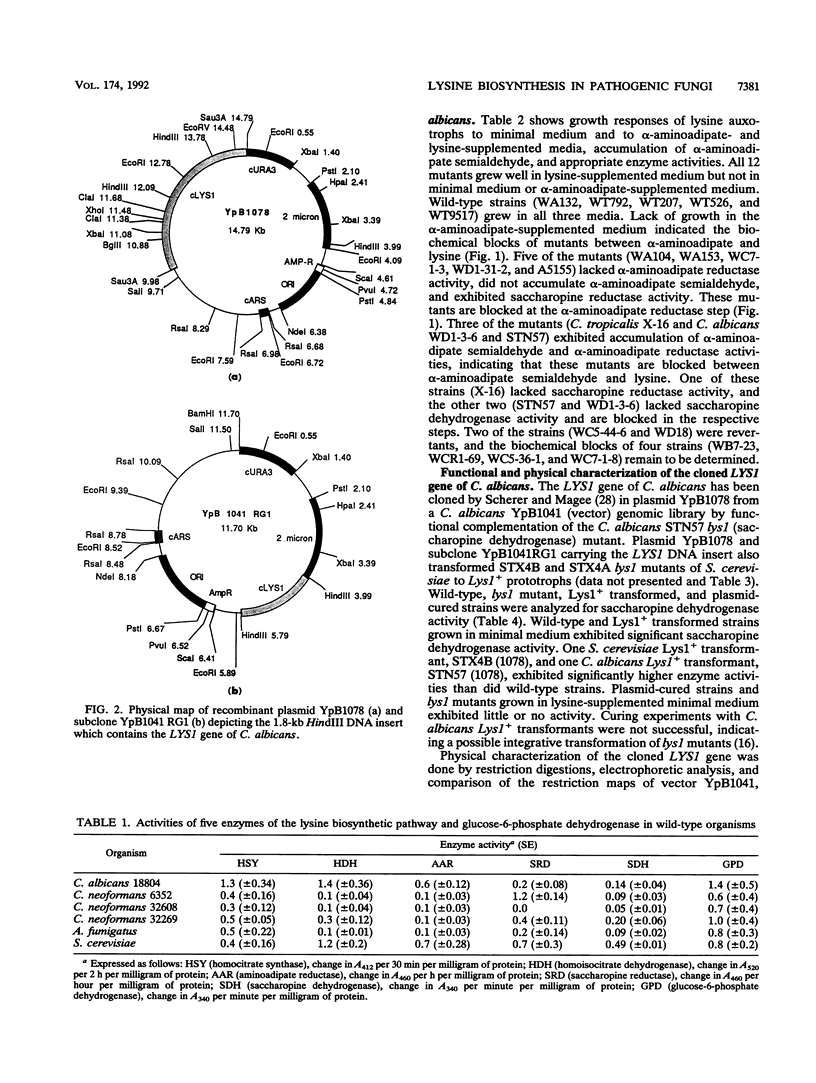
The alpha-aminoadipate pathway for the biosynthesis of lysine is present only in fungi and euglena. Until now, this unique metabolic pathway has never been investigated in the opportunistic fungal pathogens Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus. Five of the eight enzymes (homocitrate synthase, homoisocitrate dehydrogenase, alpha-aminoadipate reductase, saccharopine reductase, and saccharopine dehydrogenase) of the alpha-aminoadipate pathway and glucose-6-phosphate dehydrogenase, a glycolytic enzyme used as a control, were demonstrated in wild-type cells of these organisms. All enzymes were present in Saccharomyces cerevisiae and the pathogenic organisms except C. neoformans 32608 serotype C, which exhibited no saccharopine reductase activity. The levels of enzyme activity varied considerably from strain to strain. Variation among organisms was also observed for the control enzyme. Among the pathogens, C. albicans exhibited much higher homocitrate synthase, homoisocitrate dehydrogenase, and alpha-aminoadipate reductase activities. Seven lysine auxotrophs of C. albicans and one of Candida tropicalis were characterized biochemically to determine the biochemical blocks and gene-enzyme relationships. Growth responses to alpha-aminoadipate- and lysine-supplemented media, accumulation of alpha-aminoadipate semialdehyde, and the lack of enzyme activity revealed that five of the mutants (WA104, WA153, WC7-1-3, WD1-31-2, and A5155) were blocked at the alpha-aminoadipate reductase step, two (STN57 and WD1-3-6) were blocked at the saccharopine dehydrogenase step, and the C. tropicalis mutant (X-16) was blocked at the saccharopine reductase step. The cloned LYS1 gene of C. albicans in the recombinant plasmid YpB1078 complemented saccharopine dehydrogenase (lys1) mutants of S. cerevisiae and C. albicans. The Lys1+ transformed strains exhibited significant saccharopine dehydrogenase activity in comparison with untransformed mutants. The cloned LYS1 gene has been localized on a 1.8-kb HindIII DNA insert of the recombinant plasmid YpB1041RG1. These results established the gene-enzyme relationship in the second half of the alpha-aminoadipate pathway. The presence of this unique pathway in the pathogenic fungi could be useful for their rapid detection and control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altboum Z., Gottlieb S., Lebens G. A., Polacheck I., Segal E. Isolation of the Candida albicans histidinol dehydrogenase (HIS4) gene and characterization of a histidine auxotroph. J Bacteriol. 1990 Jul;172(7):3898–3904. doi: 10.1128/jb.172.7.3898-3904.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee J. K., Strassman M. Accumulation of tricarboxylic acids related to lysine biosynthesis in a yeast mutant. J Biol Chem. 1967 May 25;242(10):2542–2546. [PubMed] [Google Scholar]
- Bodey G. P. The emergence of fungi as major hospital pathogens. J Hosp Infect. 1988 Feb;11 (Suppl A):411–426. doi: 10.1016/0195-6701(88)90220-4. [DOI] [PubMed] [Google Scholar]
- Borell C. W., Urrestarazu L. A., Bhattacharjee J. K. Two unlinked lysine genes (LYS9 and LYS14) are required for the synthesis of saccharopine reductase in Saccharomyces cerevisiae. J Bacteriol. 1984 Jul;159(1):429–432. doi: 10.1128/jb.159.1.429-432.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Fink G. R. Yeast: an experimental organism for modern biology. Science. 1988 Jun 10;240(4858):1439–1443. doi: 10.1126/science.3287619. [DOI] [PubMed] [Google Scholar]
- Gaillardin C. M., Ribet A. M., Heslot H. Wild-type and mutant forms of homoisocitric dehydrogenase in the yeast Saccharomycopsis lipolytica. Eur J Biochem. 1982 Nov 15;128(2-3):489–494. doi: 10.1111/j.1432-1033.1982.tb06991.x. [DOI] [PubMed] [Google Scholar]
- Glass J., Bhattacharjee J. K. Biosynthesis of lysine in Rhodotorula: accumulation of homocitric, homoaconitic, and homoisocitric acids in a leaky mutant. Genetics. 1971 Mar;67(3):365–376. doi: 10.1093/genetics/67.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES E. E., BROQUIST H. P. SACCHAROPINE, AN INTERMEDIATE OF THE AMINOADIPIC ACID PATHWAY OF LYSINE BIOSYNTHESIS. II. STUDIES IN SACCHAROMYCES CEREVISEAE. J Biol Chem. 1965 Jun;240:2531–2536. [PubMed] [Google Scholar]
- Jaklitsch W. M., Kubicek C. P. Homocitrate synthase from Penicillium chrysogenum. Localization, purification of the cytosolic isoenzyme, and sensitivity to lysine. Biochem J. 1990 Jul 1;269(1):247–253. doi: 10.1042/bj2690247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G., Bode R., Schmidt H., Samsonova I. A., Birnbaum D. Identification of a lys2 mutant of Candida maltosa by means of transformation. Curr Genet. 1987;11(5):385–391. doi: 10.1007/BF00378181. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Kirsch D. R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986 Jan;6(1):142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., Koltin Y., Gorman J. A., Magee P. T. Assignment of cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol Cell Biol. 1988 Nov;8(11):4721–4726. doi: 10.1128/mcb.8.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae, edition 9. Microbiol Rev. 1985 Sep;49(3):181–213. doi: 10.1128/mr.49.3.181-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musial C. E., Cockerill F. R., 3rd, Roberts G. D. Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev. 1988 Oct;1(4):349–364. doi: 10.1128/cmr.1.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Candida infections: an overview. Crit Rev Microbiol. 1987;15(1):1–5. doi: 10.3109/10408418709104444. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Lebens G. A. Electrophoretic karyotype of the pathogenic yeast Cryptococcus neoformans. J Gen Microbiol. 1989 Jan;135(1):65–71. doi: 10.1099/00221287-135-1-65. [DOI] [PubMed] [Google Scholar]
- Ramos F., Dubois E., Piérard A. Control of enzyme synthesis in the lysine biosynthetic pathway of Saccharomyces cerevisiae. Evidence for a regulatory role of gene LYS14. Eur J Biochem. 1988 Jan 15;171(1-2):171–176. doi: 10.1111/j.1432-1033.1988.tb13773.x. [DOI] [PubMed] [Google Scholar]
- Sarachek A., Rhoads D. D., Schwarzhoff R. H. Hybridization of Candida albicans through fusion of protoplasts. Arch Microbiol. 1981 Mar;129(1):1–8. doi: 10.1007/BF00417169. [DOI] [PubMed] [Google Scholar]
- Scherer S., Magee P. T. Genetics of Candida albicans. Microbiol Rev. 1990 Sep;54(3):226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd M. G. Pathogenicity of morphological and auxotrophic mutants of Candida albicans in experimental infections. Infect Immun. 1985 Nov;50(2):541–544. doi: 10.1128/iai.50.2.541-544.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storts D. R., Bhattacharjee J. K. Properties of revertants of lys2 and lys5 mutants as well as alpha-aminoadipate-semialdehyde dehydrogenase from Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1989 May 30;161(1):182–186. doi: 10.1016/0006-291x(89)91578-7. [DOI] [PubMed] [Google Scholar]
- Strassman M., Ceci L. N. Enzymatic formation of alpha-ketoadipic acid from homoisocitric acid. J Biol Chem. 1965 Nov;240(11):4357–4361. [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Wang L., Okamoto S., Bhattacharjee J. K. Cloning and physical characterization of linked lysine genes (lys4, lys15) of Saccharomyces cerevisiae. Curr Genet. 1989 Jul;16(1):7–12. doi: 10.1007/BF00411077. [DOI] [PubMed] [Google Scholar]
- Winston M. K., Bhattacharjee J. K. Growth inhibition by alpha-aminoadipate and reversal of the effect by specific amino acid supplements in Saccharomyces cerevisiae. J Bacteriol. 1982 Nov;152(2):874–879. doi: 10.1128/jb.152.2.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. H., Bhattacharjee J. K. Lysine biosynthesis pathway and biochemical blocks of lysine auxotrophs of Schizosaccharomyces pombe. J Bacteriol. 1988 Dec;170(12):5968–5970. doi: 10.1128/jb.170.12.5968-5970.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. H., Garrad R. C., Winston M. K., Bhattacharjee J. K. Use of alpha-aminoadipate and lysine as sole nitrogen source by Schizosaccharomyces pombe and selected pathogenic fungi. J Basic Microbiol. 1991;31(2):149–156. doi: 10.1002/jobm.3620310215. [DOI] [PubMed] [Google Scholar]