Abstract
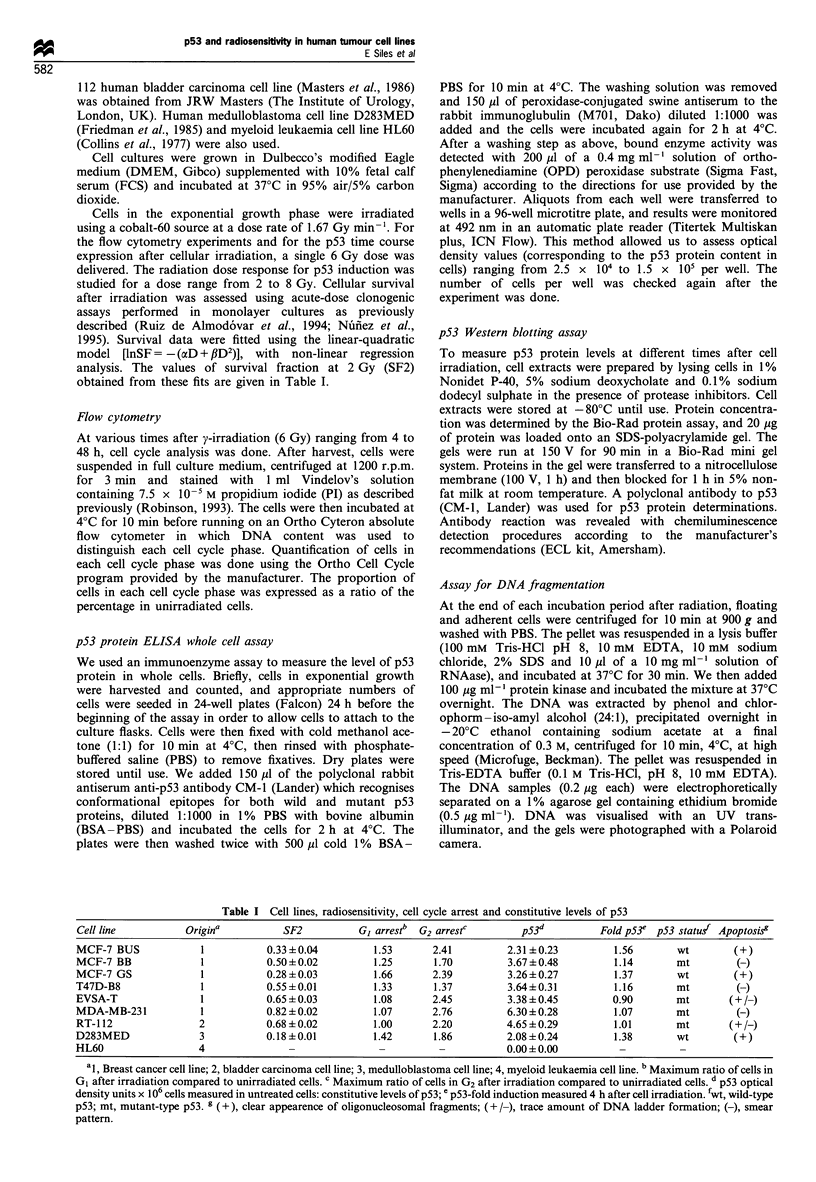
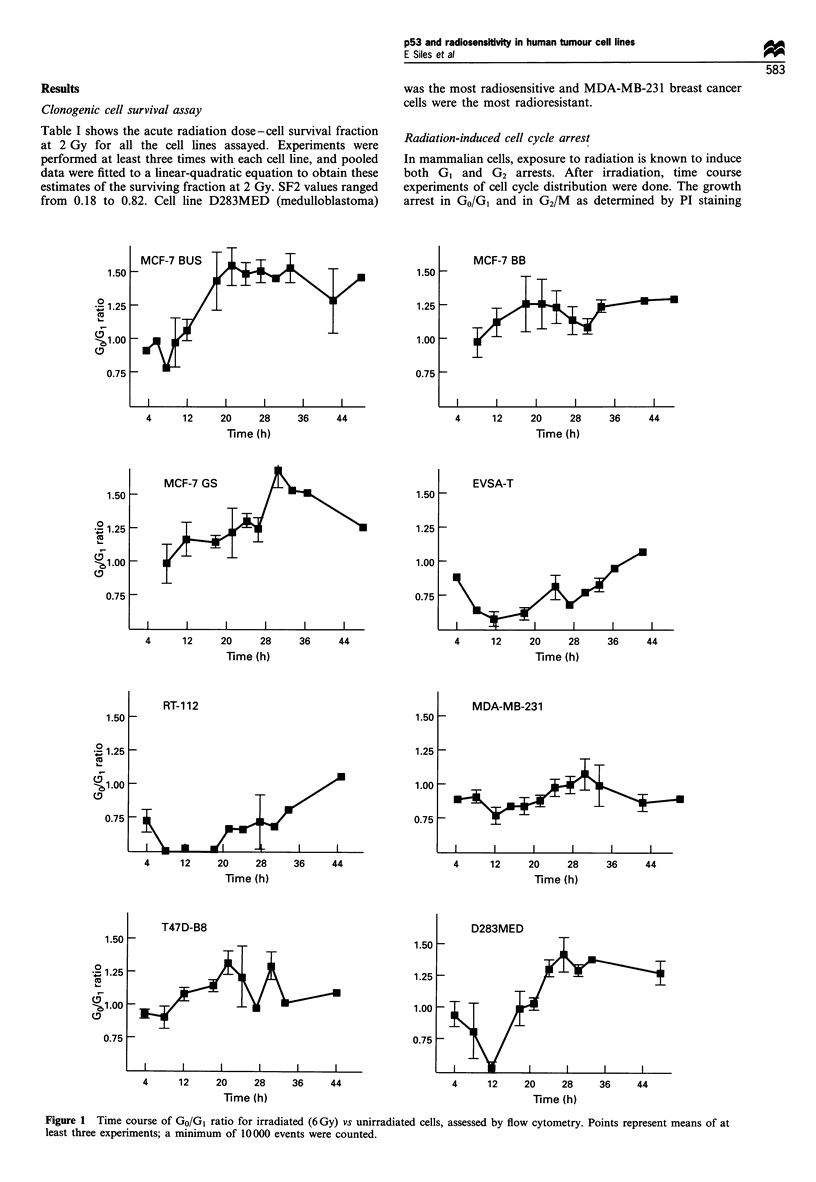
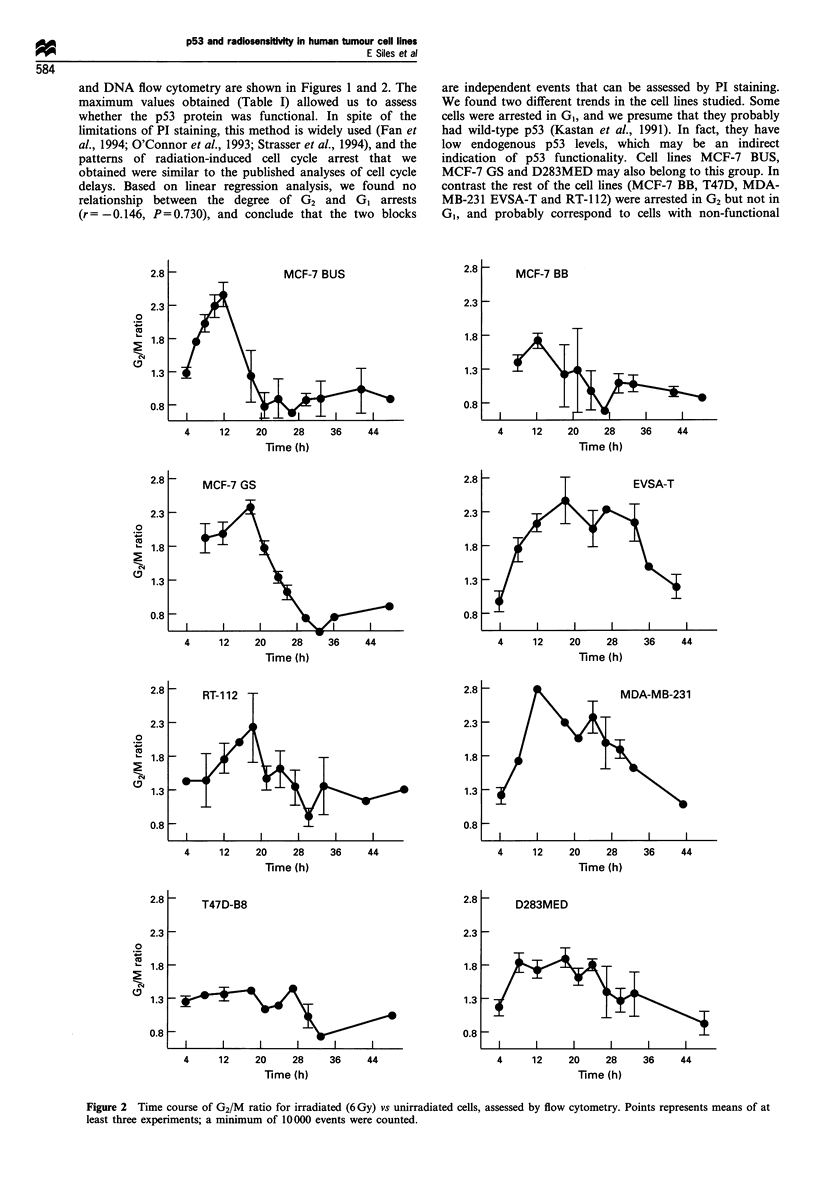
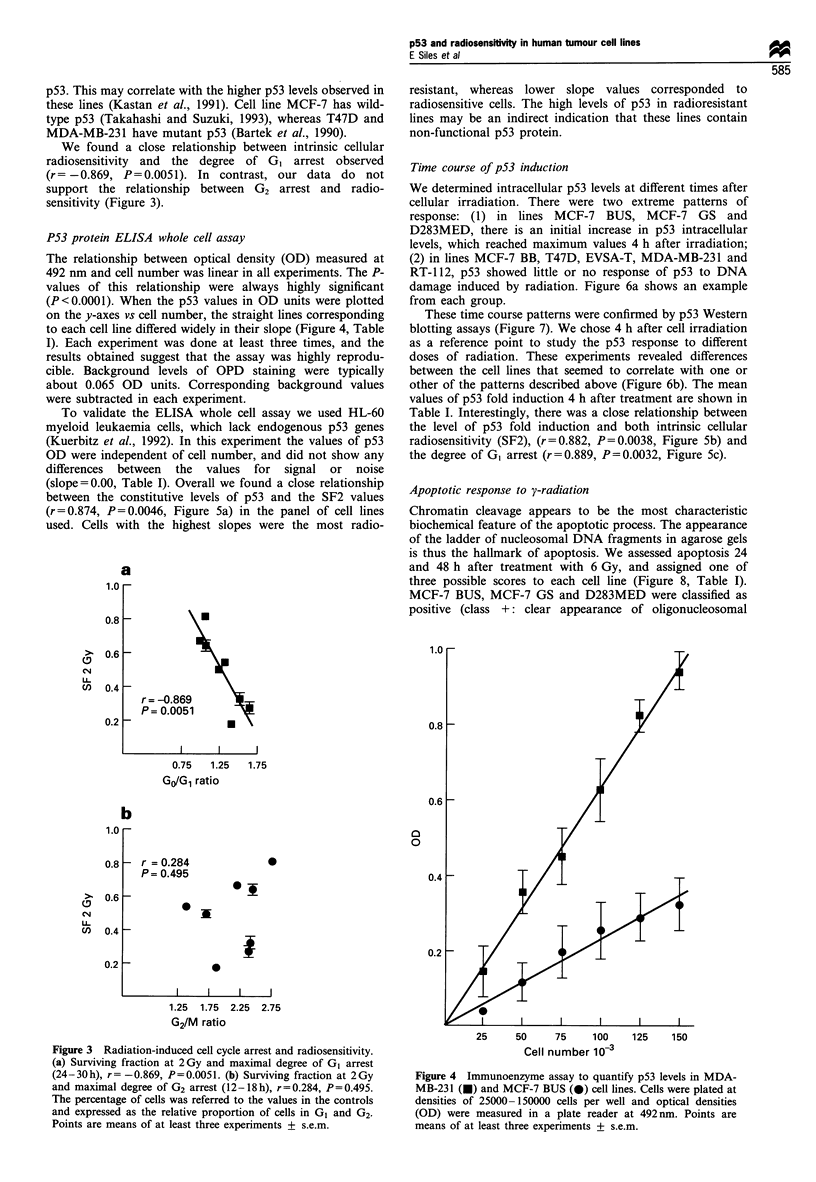
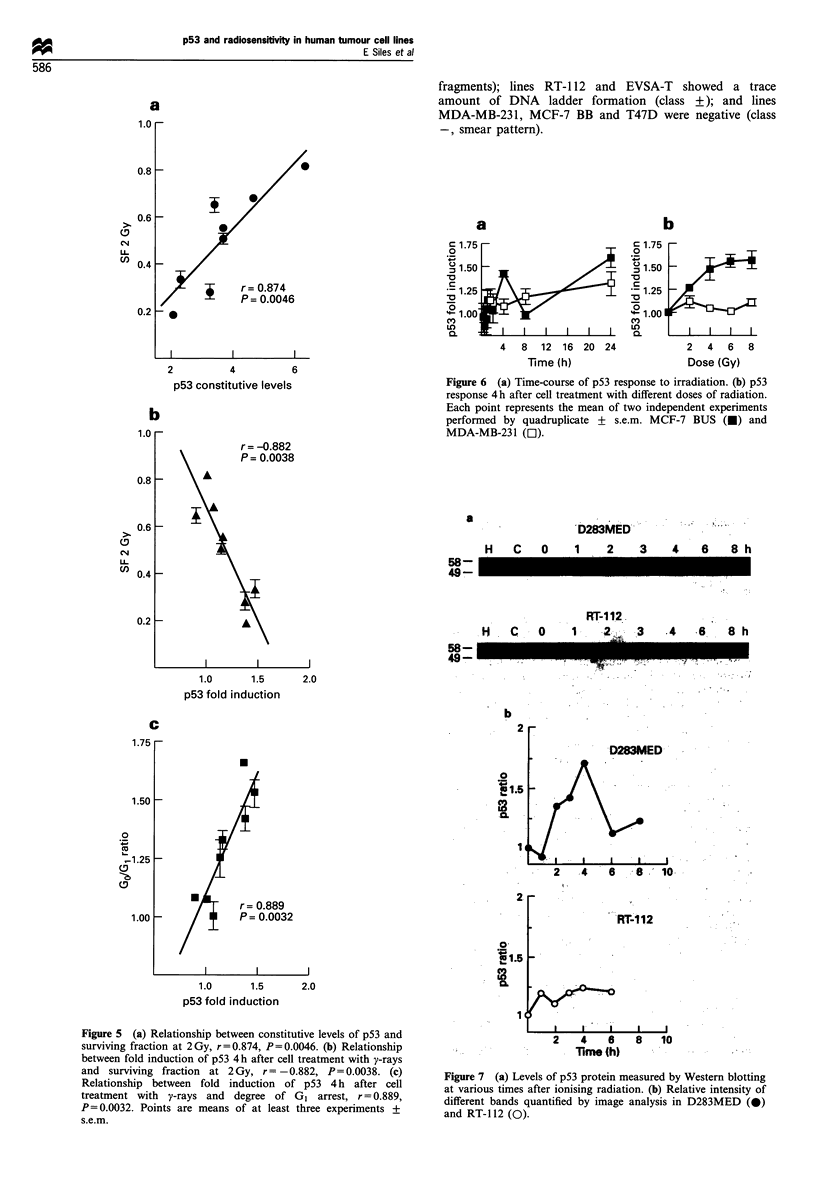
We examined the relationship between p53 levels before and after irradiation, radiation-induced cell cycle delays, apoptotic cell death and radiosensitivity in a panel of eight human tumour cell lines. The cell lines differed widely in their clonogenic survival after radiation, (surviving fraction at 2 Gy: SF2=0.18-0.82). Constitutive p53 protein levels varied from 2.2 +/- 0.4 to 6.3 +/- 0.3 optical density units (OD) per 10(6) cells. p53 after irradiation (6 Gy) also varied between the cell lines, ranging from no induction to a 1.6-fold increase in p53 levels 4 h after treatment. p53 function was also assessed by G1 cell cycle arrest after irradiation. The cellular response to radiation, measured as G0/G1 arrest, and the induction of apoptosis were in good agreement. However, a trace amount of DNA ladder formation was found in two cell lines lacking G1 arrest. Overall cellular radiosensitivity correlated well with the level of radiation-induced G1 arrest (correlation coefficient r=0.856; P=0.0067), with p53 constitutive levels (r=0.874, P=0.0046), and with p53 protein fold induction (r=-0.882, P=0.0038). Our data suggest that (1) the constitutive p53 level, (2) G1 arrest after irradiation, or (3) the p53 protein response to radiation may be good predictive tests for radiosensitivity in some cell types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Bracey T. S., Miller J. C., Preece A., Paraskeva C. Gamma-radiation-induced apoptosis in human colorectal adenoma and carcinoma cell lines can occur in the absence of wild type p53. Oncogene. 1995 Jun 15;10(12):2391–2396. [PubMed] [Google Scholar]
- Cailleau R., Young R., Olivé M., Reeves W. J., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974 Sep;53(3):661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman C. E., Wolff A. C., Chen C. Y., Fornace A. J., Jr, Kastan M. B. The p53-dependent G1 cell cycle checkpoint pathway and ataxia-telangiectasia. Cancer Res. 1994 Oct 1;54(19):5054–5058. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Fan S., el-Deiry W. S., Bae I., Freeman J., Jondle D., Bhatia K., Fornace A. J., Jr, Magrath I., Kohn K. W., O'Connor P. M. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994 Nov 15;54(22):5824–5830. [PubMed] [Google Scholar]
- Friedman H. S., Burger P. C., Bigner S. H., Trojanowski J. Q., Wikstrand C. J., Halperin E. C., Bigner D. D. Establishment and characterization of the human medulloblastoma cell line and transplantable xenograft D283 Med. J Neuropathol Exp Neurol. 1985 Nov;44(6):592–605. doi: 10.1097/00005072-198511000-00005. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Ray A., Lemoine N. R., Midgley C. A., Krausz T., Lane D. P. p53 immunostaining as a marker of malignant disease in diagnostic cytopathology. Lancet. 1991 Aug 24;338(8765):513–513. doi: 10.1016/0140-6736(91)90586-e. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Perry M. E., Chang A., Silver A., Dittmer D., Wu M., Welsh D. The 1993 Walter Hubert Lecture: the role of the p53 tumour-suppressor gene in tumorigenesis. Br J Cancer. 1994 Mar;69(3):409–416. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Masters J. R., Hepburn P. J., Walker L., Highman W. J., Trejdosiewicz L. K., Povey S., Parkar M., Hill B. T., Riddle P. R., Franks L. M. Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines. Cancer Res. 1986 Jul;46(7):3630–3636. [PubMed] [Google Scholar]
- McIlwrath A. J., Vasey P. A., Ross G. M., Brown R. Cell cycle arrests and radiosensitivity of human tumor cell lines: dependence on wild-type p53 for radiosensitivity. Cancer Res. 1994 Jul 15;54(14):3718–3722. [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Kemp C. J., Hickman J. A., Balmain A., Lane D. P., Hall P. A. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994 Feb 1;54(3):614–617. [PubMed] [Google Scholar]
- Nagasawa H., Keng P., Harley R., Dahlberg W., Little J. B. Relationship between gamma-ray-induced G2/M delay and cellular radiosensitivity. Int J Radiat Biol. 1994 Oct;66(4):373–379. doi: 10.1080/09553009414551311. [DOI] [PubMed] [Google Scholar]
- Núez M. I., Villalobos M., Olea N., Valenzuela M. T., Pedraza V., McMillan T. J., Ruiz de Almodóvar J. M. Radiation-induced DNA double-strand break rejoining in human tumour cells. Br J Cancer. 1995 Feb;71(2):311–316. doi: 10.1038/bjc.1995.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor P. M., Jackman J., Jondle D., Bhatia K., Magrath I., Kohn K. W. Role of the p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt's lymphoma cell lines. Cancer Res. 1993 Oct 15;53(20):4776–4780. [PubMed] [Google Scholar]
- Powell S. N., McMillan T. J. The repair fidelity of restriction enzyme-induced double strand breaks in plasmid DNA correlates with radioresistance in human tumor cell lines. Int J Radiat Oncol Biol Phys. 1994 Jul 30;29(5):1035–1040. doi: 10.1016/0360-3016(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodóvar J. M., Núez M. I., McMillan T. J., Olea N., Mort C., Villalobos M., Pedraza V., Steel G. G. Initial radiation-induced DNA damage in human tumour cell lines: a correlation with intrinsic cellular radiosensitivity. Br J Cancer. 1994 Mar;69(3):457–462. doi: 10.1038/bjc.1994.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichtholz B., Legros Y., Gillet D., Gaillard C., Marty M., Lane D., Calvo F., Soussi T. The immune response to p53 in breast cancer patients is directed against immunodominant epitopes unrelated to the mutational hot spot. Cancer Res. 1992 Nov 15;52(22):6380–6384. [PubMed] [Google Scholar]
- Slichenmyer W. J., Nelson W. G., Slebos R. J., Kastan M. B. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993 Sep 15;53(18):4164–4168. [PubMed] [Google Scholar]
- Soto A. M., Murai J. T., Siiteri P. K., Sonnenschein C. Control of cell proliferation: evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res. 1986 May;46(5):2271–2275. [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Stewart N., Hicks G. G., Paraskevas F., Mowat M. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 1995 Jan 5;10(1):109–115. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Jacks T., Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994 Oct 21;79(2):329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Suzuki K. Association of insulin-like growth-factor-I-induced DNA synthesis with phosphorylation and nuclear exclusion of p53 in human breast cancer MCF-7 cells. Int J Cancer. 1993 Sep 30;55(3):453–458. doi: 10.1002/ijc.2910550322. [DOI] [PubMed] [Google Scholar]
- Whitaker S. J., Ung Y. C., McMillan T. J. DNA double-strand break induction and rejoining as determinants of human tumour cell radiosensitivity. A pulsed-field gel electrophoresis study. Int J Radiat Biol. 1995 Jan;67(1):7–18. doi: 10.1080/09553009514550021. [DOI] [PubMed] [Google Scholar]
- Wurm R., Burnet N. G., Duggal N., Yarnold J. R., Peacock J. H. Cellular radiosensitivity and DNA damage in primary human fibroblasts. Int J Radiat Oncol Biol Phys. 1994 Oct 15;30(3):625–633. doi: 10.1016/0360-3016(92)90949-i. [DOI] [PubMed] [Google Scholar]
- Xia F., Wang X., Wang Y. H., Tsang N. M., Yandell D. W., Kelsey K. T., Liber H. L. Altered p53 status correlates with differences in sensitivity to radiation-induced mutation and apoptosis in two closely related human lymphoblast lines. Cancer Res. 1995 Jan 1;55(1):12–15. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]




