Abstract
Some oral cancers are preceded by premalignant lesions which include leucoplakia and erythroplakia. At present there are no reliable markers to identify lesions that may progress to malignancy. We have analysed 30 potentially malignant oral lesions for deletions at chromosomal regions that harbour tumour-suppressor genes for oral cancer. A total of 16 of 30 cases (53%) showed loss of heterozygosity (LOH) or allele imbalance at TP53, DCC, 3p21.3-22.1 or 3p12.1-13. These genetic alterations were detected in dysplastic lesions but not in histologically normal mucosa and may be early events in the carcinogenic process. A total of 64% of dysplastic lesions that recurred during the study showed LOH or allele imbalance in the initial biopsy and the number of genetic abnormalities increased in the tumours that developed. This type of molecular profiling may help to identify patients with lesions that may recur or acquire additional genetic events and progress to malignancy.
Full text
PDF


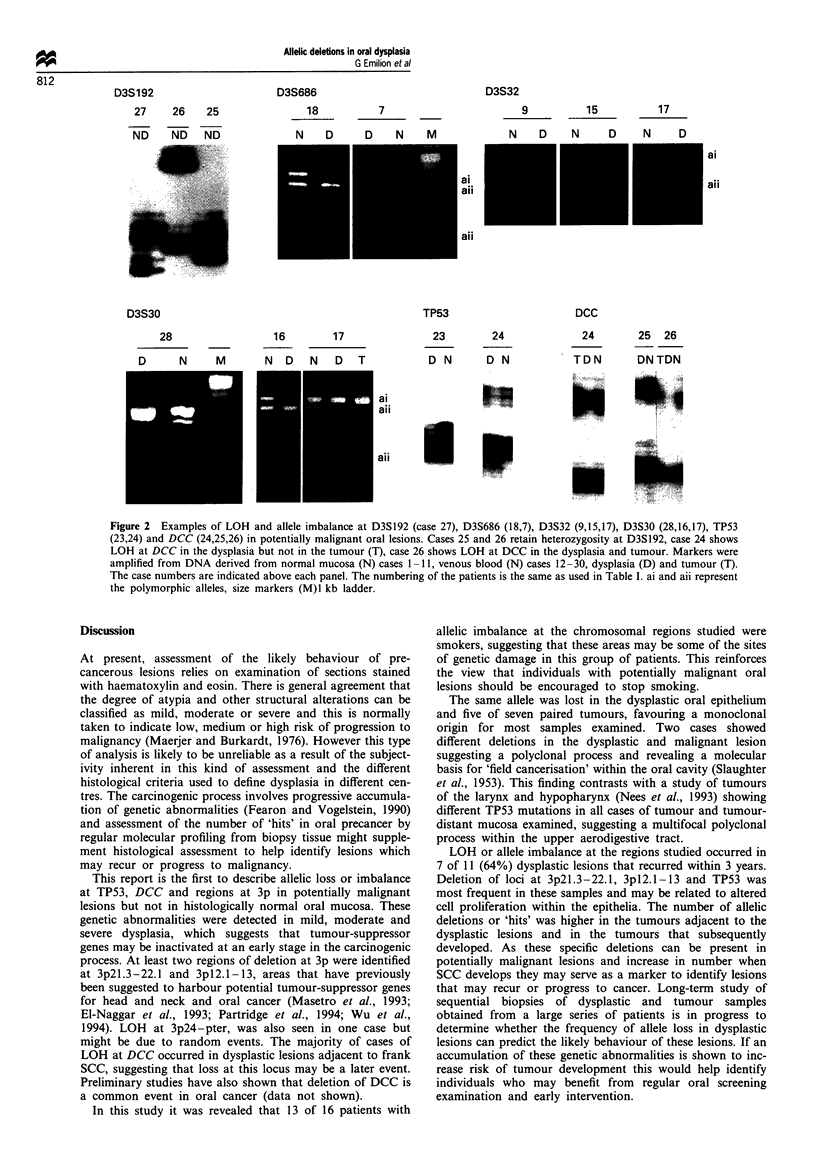

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. O., Hakim J., Koch W., van der Riet P., Hruban R. H., Roa R. A., Correo R., Eby Y. J., Ruppert J. M., Sidransky D. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993 Oct 1;53(19):4477–4480. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res. 1991 Sep 15;51(18 Suppl):5023s–5044s. [PubMed] [Google Scholar]
- Nees M., Homann N., Discher H., Andl T., Enders C., Herold-Mende C., Schuhmann A., Bosch F. X. Expression of mutated p53 occurs in tumor-distant epithelia of head and neck cancer patients: a possible molecular basis for the development of multiple tumors. Cancer Res. 1993 Sep 15;53(18):4189–4196. [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Partridge M., Kiguwa S., Langdon J. D. Frequent deletion of chromosome 3p in oral squamous cell carcinoma. Eur J Cancer B Oral Oncol. 1994 Jul;30B(4):248–251. doi: 10.1016/0964-1955(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Renan M. J. How many mutations are required for tumorigenesis? Implications from human cancer data. Mol Carcinog. 1993;7(3):139–146. doi: 10.1002/mc.2940070303. [DOI] [PubMed] [Google Scholar]
- SLAUGHTER D. P., SOUTHWICK H. W., SMEJKAL W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953 Sep;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Ganly P., Hasleton P., Rudd R., Sinha G., Bleehen N. M., Rabbitts P. p53 and chromosome 3 abnormalities, characteristic of malignant lung tumours, are detectable in preinvasive lesions of the bronchus. Oncogene. 1992 Oct;7(10):1989–1997. [PubMed] [Google Scholar]
- Wu C. L., Sloan P., Read A. P., Harris R., Thakker N. Deletion mapping on the short arm of chromosome 3 in squamous cell carcinoma of the oral cavity. Cancer Res. 1994 Dec 15;54(24):6484–6488. [PubMed] [Google Scholar]
- el-Naggar A. K., Lee M. S., Wang G., Luna M. A., Goepfert H., Batsakis J. G. Polymerase chain reaction-based restriction fragment length polymorphism analysis of the short arm of chromosome 3 in primary head and neck squamous carcinoma. Cancer. 1993 Aug 1;72(3):881–886. doi: 10.1002/1097-0142(19930801)72:3<881::aid-cncr2820720337>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- van der Riet P., Nawroz H., Hruban R. H., Corio R., Tokino K., Koch W., Sidransky D. Frequent loss of chromosome 9p21-22 early in head and neck cancer progression. Cancer Res. 1994 Mar 1;54(5):1156–1158. [PubMed] [Google Scholar]



