Abstract
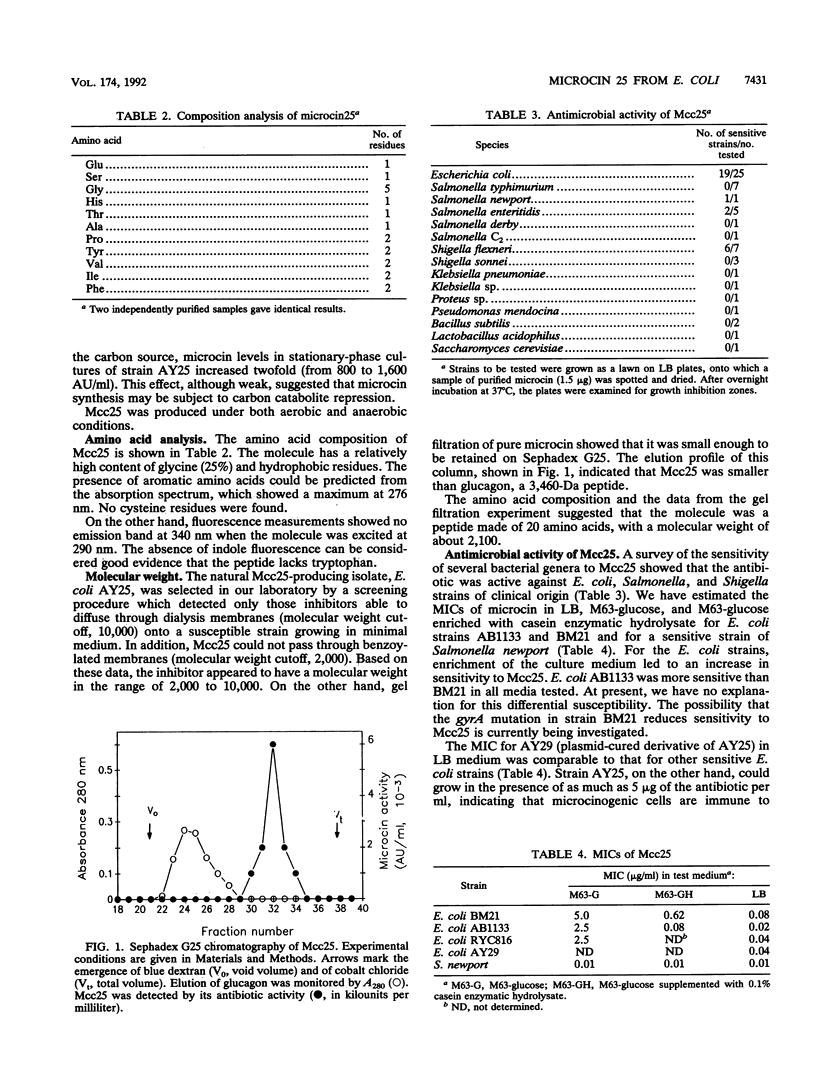
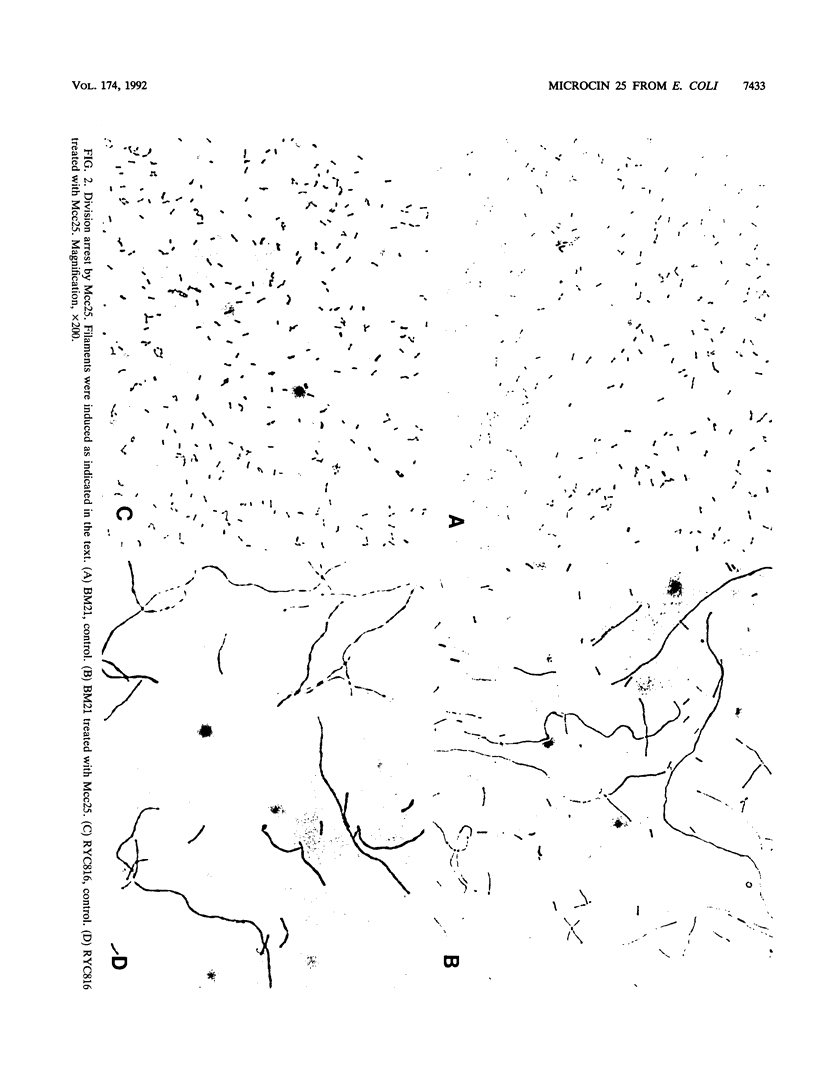
Microcin 25, a peptide antibiotic excreted by an Escherichia coli strain isolated from human feces, was purified to homogeneity and characterized. Composition analysis and data from gel filtration indicated that microcin 25 may contain 20 amino acid residues. It has a blocked amino-terminal end. Microcin synthesis and immunity are plasmid determined, and the antibiotic was produced in minimal medium when the cultures entered the stationary phase of growth. The peptide appears to interfere with cell division, since susceptible cells filamented when exposed to it. This response does not seem to be mediated by the SOS system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y. Nitrogen metabolite regulation of antibiotic biosynthesis. Annu Rev Microbiol. 1980;34:209–233. doi: 10.1146/annurev.mi.34.100180.001233. [DOI] [PubMed] [Google Scholar]
- Asensio C., Pérez-Díaz J. C. A new family of low molecular weight antibiotics from enterobacteria. Biochem Biophys Res Commun. 1976 Mar 8;69(1):7–14. doi: 10.1016/s0006-291x(76)80264-1. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Braun V., Schmitz G. Excretion of a protease by Serratia marcescens. Arch Microbiol. 1980 Jan;124(1):55–61. doi: 10.1007/BF00407028. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E., Sowell M. O. Synthesis of penicillin-binding protein 6 by stationary-phase Escherichia coli. J Bacteriol. 1982 Jul;151(1):491–494. doi: 10.1128/jb.151.1.491-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Dassa E., Cahu M., Desjoyaux-Cherel B., Boquet P. L. The acid phosphatase with optimum pH of 2.5 of Escherichia coli. Physiological and Biochemical study. J Biol Chem. 1982 Jun 25;257(12):6669–6676. [PubMed] [Google Scholar]
- Davagnino J., Herrero M., Furlong D., Moreno F., Kolter R. The DNA replication inhibitor microcin B17 is a forty-three-amino-acid protein containing sixty percent glycine. Proteins. 1986 Nov;1(3):230–238. doi: 10.1002/prot.340010305. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. Genes and the replication cycle of Escherichia coli. Res Microbiol. 1990 Jan;141(1):64–75. doi: 10.1016/0923-2508(90)90099-c. [DOI] [PubMed] [Google Scholar]
- García-Bustos J. F., Pezzi N., Asensio C. Microcin 7: purification and properties. Biochem Biophys Res Commun. 1984 Mar 15;119(2):779–785. doi: 10.1016/s0006-291x(84)80318-6. [DOI] [PubMed] [Google Scholar]
- Garrido M. C., Herrero M., Kolter R., Moreno F. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 1988 Jun;7(6):1853–1862. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givskov M., Olsen L., Molin S. Cloning and expression in Escherichia coli of the gene for extracellular phospholipase A1 from Serratia liquefaciens. J Bacteriol. 1988 Dec;170(12):5855–5862. doi: 10.1128/jb.170.12.5855-5862.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Genetic and physiological characterization of Escherichia coli mutants deficient in phosphoenolpyruvate carboxykinase activity. J Bacteriol. 1980 Mar;141(3):1115–1121. doi: 10.1128/jb.141.3.1115-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Chico C., San Millán J. L., Kolter R., Moreno F. Growth phase and ompR regulation of transcription of microcin B17 genes. J Bacteriol. 1986 Sep;167(3):1058–1065. doi: 10.1128/jb.167.3.1058-1065.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M., Moreno F. Microcin B17 blocks DNA replication and induces the SOS system in Escherichia coli. J Gen Microbiol. 1986 Feb;132(2):393–402. doi: 10.1099/00221287-132-2-393. [DOI] [PubMed] [Google Scholar]
- Laviña M., Gaggero C., Moreno F. Microcin H47, a chromosome-encoded microcin antibiotic of Escherichia coli. J Bacteriol. 1990 Nov;172(11):6585–6588. doi: 10.1128/jb.172.11.6585-6588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. Regulation of cell division in E. coli. Trends Genet. 1990 Jan;6(1):22–25. doi: 10.1016/0168-9525(90)90045-8. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control of antibiotic biosynthesis. Microbiol Rev. 1980 Jun;44(2):230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Wachi M., Ishino F. Machinery for cell growth and division: penicillin-binding proteins and other proteins. Res Microbiol. 1990 Jan;141(1):89–103. doi: 10.1016/0923-2508(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr Purification and characterization of pro-TraTp, the signal sequence-containing precursor of a secreted protein encoded by the F sex factor. J Bacteriol. 1984 May;158(2):464–473. doi: 10.1128/jb.158.2.464-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa M. A., Díaz-Guerra L., San Millán J. L., Moreno F. Cloning and mapping of the genetic determinants for microcin C7 production and immunity. J Bacteriol. 1986 Dec;168(3):1384–1391. doi: 10.1128/jb.168.3.1384-1391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Rodriguez R. L., Preiss J. Biosynthesis of bacterial glycogen. Cloning of the glycogen biosynthetic enzyme structural genes of Escherichia coli. J Biol Chem. 1981 Jul 10;256(13):6944–6952. [PubMed] [Google Scholar]
- Pugsley A. P. The ins and outs of colicins. Part I: Production, and translocation across membranes. Microbiol Sci. 1984 Oct;1(7):168–175. [PubMed] [Google Scholar]
- SCHRAM E., MOORE S., BIGWOOD E. J. Chromatographic determination of cystine as cysteic acid. Biochem J. 1954 May;57(1):33–37. doi: 10.1042/bj0570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Millán J. L., Kolter R., Moreno F. Plasmid genes required for microcin B17 production. J Bacteriol. 1985 Sep;163(3):1016–1020. doi: 10.1128/jb.163.3.1016-1020.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Garen A. Fragments of alkaline phosphatase from nonsense mutants. I. Isolation and characterization of fragments from amber and ochre mutants. J Mol Biol. 1969 Nov 14;45(3):549–566. doi: 10.1016/0022-2836(69)90312-x. [DOI] [PubMed] [Google Scholar]