Abstract
Proteins necessary for maintenance and function of eukaryotic flagella are synthesized in the cell body. Transport of the inner dynein arm subunit p28IDA4 in Chlamydomonas flagella requires the activity of the kinesin KHP1FLA10, a protein inactive at restrictive temperature in fla10, a temperature-dependent mutant of flagellar assembly. To identify other molecules involved in active transport of inner dynein arms within flagella we searched for polypeptides of the cytoplasmic matrix of flagella that fulfill two conditions: they bind to p28 and require the activity of KHP1 to be present in flagella. We found that the cytoplasmic matrix of flagella contains a previously unidentified “17S” complex of at least 13 polypeptides that in part is associated with p28. The 17S complex is present at permissive but not at restrictive temperature in fla10 flagella. It also turns over in the cytoplasmic matrix more frequently than dynein arms within the axoneme. This evidence suggests that the 17S complex transports precursors of inner dynein arms within flagella.
Intracellular transport of protein complexes, or membrane-bound vesicles, often requires the participation of microtubules and the activity of molecular motors, such as dyneins and kinesins (1). Molecular motors are recruited in specific cellular compartments and display their activity by moving a cargo along the microtubules. The questions of whether the motors themselves are transported or diffuse toward their final site of activity are relevant for understanding the dynamics of intracellular and microtubule-based transport. We addressed these questions by the analysis of Chlamydomonas flagella, a model system for microtubule-based movement (2, 3) that can be dissected by genetics (4, 5).
Our previous work was focused on the inner dynein arms, a group of six dyneins (6) binding to doublet microtubules in the internal part of the axonemal shaft. The inner dynein arms, but not the outer dynein arms, are required for the formation of both ciliary and flagellar type of waveforms of flagella (7). They are located in asymmetric positions along (8) and around (9) the axoneme. Some of them bind to microtubules at different times during flagellar assembly (8).
Knowledge of both subunit composition and axonemal location of inner dynein arms allowed us to address the question of whether the dyneins themselves are transported toward their final site of activity (10). To identify the mode of transport of a subset of inner dynein arms within flagella we recently analyzed the movement of p28IDA4 (6), a light chain of two types of inner dynein arms. p28, but not the outer dynein arm subunit IC69ODA6 (11), requires the activity of the kinesin homologous protein 1, KHP1FLA10 (12), to reach the distal part of flagella (10).
KHP1 carries an ATP binding site (12, 13) and presumably moves over outer doublet microtubules and underneath the membrane during the transport of a cargo within the cytoplasmic matrix of flagella (14). The cargo may consist of large protein complexes that appear as granule-like particles and move bidirectionally within flagella (15). Therefore, p28 may bind to KHP1 through intervening proteins that form large complexes.
To further develop the study of active transport of inner dynein arms in the flagellar compartment we searched for other components of the machinery transporting p28 within the cytoplasmic matrix of flagella. To this purpose we planned to identify large protein complexes of the cytoplasmic matrix that could bind p28 and require the activity of KHP1 to move in the flagellar space. To ascertain that detectable amounts of p28 are transported within flagella at the time of our analysis we isolated flagella at different states of assembly: steady-state flagella and regenerating flagella where proteins are imported from the cell body and form the axonemes within 1 h (16). We found that the cytoplasmic matrix includes a novel complex of at least 13 polypeptides independently from the state of assembly of flagella. Different properties of this complex suggest that it may be involved in active transport of precursors of inner dynein arms within flagella.
METHODS
Preparation of the Cytoplasmic Matrix.
Wild-type cells were grown and labeled in solid medium at 25°C (17). Flagella were processed as follows: (i) regenerated from 35S-labeled cells at 25°C for 1 h (8); (ii) separated from the cell body by pH shock and differential sedimentation (17); (iii) resuspended in 0.05 M NaCl, 4 mM MgCl2, and 0.01 M Hepes (pH 7.3); and (iv) exposed to 0.1% Nonidet P-40. Cytoplasmic matrix and membrane proteins were separated from the axonemes by a centrifugation at 14,000 rpm in a 5402 minicentrifuge (Eppendorf) for 30 min at 5°C. They represented ≈30% of the flagellar protein mass.
Alternatively, regenerating or steady state flagella were exposed to ultrasound using a XL Sonicator (Heat System, Farmingdale NY), setting 4, duty cycle 10, for 20 sec at 0°C. Approximately 40% of the flagellar proteins including the cytoplasmic matrix and a subset of axonemal proteins were solubilized under these conditions, whereas the flagellar membrane remained distinct, as assessed by optical microscopy.
Other Methods.
Pulse labeling of wild-type cells starved for sulfate (17) and collected in 0.2 mM SrCl2 and 0.01 M Hepes (pH 7.3) was performed for 20–30 min at 21°C by addition of 12.5 mCi (1 Ci = 37 GBq) of [35S]H2SO4 to 100 ml of culture. Labeling of flagella was interrupted by pH shock.
Proteins of extracts containing the cytoplasmic matrix of flagella were resolved by sedimentation in 5 ml sucrose gradients 5–20%, 0.05 M NaCl, 4 mM MgCl2, and 0.01 M Hepes (pH 7.3) at 30,000 rpm in a SW55 rotor (Beckman) for 14 h at 4°C. Outer and inner dynein arms extracted from the axonemes by 0.6 M NaCl and 1 mM ATP-Mg (18) were adopted as standards of sedimentation.
SDS/PAGE, two-dimensional electrophoresis of proteins, Western blot analysis, and immunoprecipitations were performed as described (6, 19).
Quantitative analysis of optical density or radioactivity of polypeptides was performed on dried gels using a Densitometer or a PhosphorImager (Molecular Dynamics).
Polyclonal antibodies specific for p28 were those described in ref. 6. A monoclonal antibody specific for Hsp70 was 3a3 (MA3–006; Affinity Bioreagents, Golden, CO) as described (20).
RESULTS
The Cytoplasmic Matrix of Flagella Includes a “17S” Complex of Polypeptides.
To determine whether the cytoplasmic matrix of flagella contains large protein complexes we prepared and analyzed extracts of flagella including the cytoplasmic matrix. We processed flagella either at the end of their regeneration (Fig. 1) or at steady state (Fig. 2). We extracted 35S-labeled soluble proteins by exposure of flagella either to a nonionic detergent or to ultrasound. In all cases we isolated a fraction of soluble proteins by sedimentation. Furthermore, we resolved components of this fraction by sedimentation in a sucrose gradient (Figs. 1a and 2a) and analyzed them by PAGE (Figs. 1b and 2b).
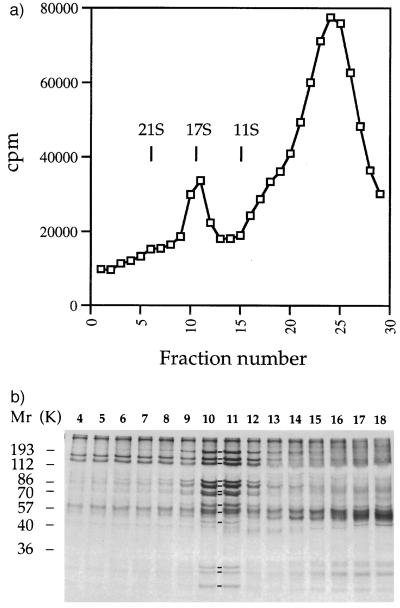
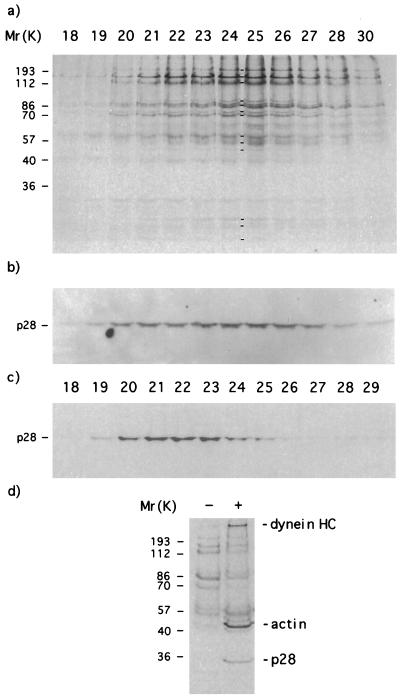
Figure 1.
A fraction of cytoplasmic matrix and membrane protein from regenerated flagella contains a 17S protein complex. (a) Sedimentation profile of 35S-labeled Nonidet P-40-soluble proteins extracted from flagella regenerated from wild-type Chlamydomonas cells. Vertical lines indicate the position of standards that were sedimented in a parallel experiment; cpm refers to 10-μl aliquots of 170-μl fractions. (b) Electrophoretograms of proteins contained in sucrose gradient fractions 4–18. Molecular weight standards are indicated on the left. Lines between lanes 10 and 11 indicate the presence of components 1–13 of the 17S complex.
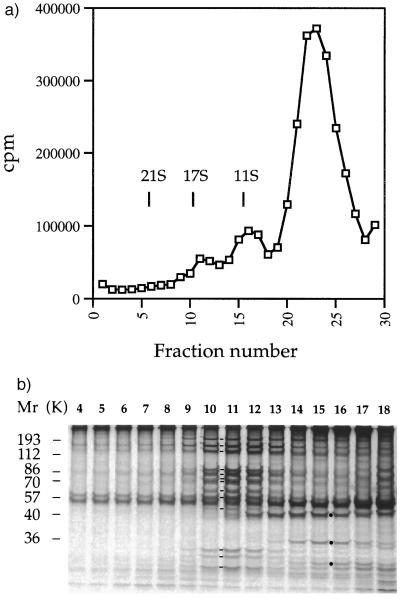
Figure 2.
A fraction of cytoplasmic matrix from steady-state flagella contains a 17S protein complex. (a) Sedimentation profile of 35S-labeled proteins extracted from flagella of wild-type Chlamydomonas cells by ultrasound. Vertical lines indicate the position of standards that were sedimented in a parallel experiment; cpm refers to 10-μl aliquots of 170-μl fractions. (b) Electrophoretograms of proteins contained in sucrose gradient fractions 4–18. Molecular weight standards are indicated on the left. Lines between lanes 10 and 11 indicate the presence of components 1–13 of the 17S complex. Dots between lanes 15 and 16 indicate inner dynein arm subunits actin, p28, and caltractin/centrin, respectively.
The sedimentation profile of proteins extracted from regenerating flagella by exposure to a nonionic detergent included two peaks (Fig. 1a). One of these peaks was formed by a complex of at least 13 polypeptides (Fig. 1b) that was not described before. The complex had an apparent sedimentation coefficient of 17 S and approximately represented 11% of the proteins resolved in the gradient and 4% of the flagellar protein mass.
The 13 polypeptides of the 17S complex were isolated and analyzed by high resolution gel electrophoresis (data not shown). They were in a molar ratio 1:1 or 1:2 relative to each other in fraction 11 (Fig. 1b). They were numbered progressively in the order of their decreasing apparent molecular weight as follows: (1) 230 kDa, (2) 165 kDa, (3) 140 kDa, (4) 94 kDa, (5) 87 kDa, (6) 75 kDa, (7) 75 kDa, (8) 64 kDa, (9) 59 kDa, (10) 51 kDa, (11) 30 kDa, (12) 27 kDa, and (13) 25 kDa. We will refer to this group of 13 polypeptides as the “17S” complex. The 17S complex may be formed by a higher number of polypeptides if some of the subunits are not resolved by the electrophoretic method that we adopted for the analysis.
To determine whether the presence of the 17S complex in extracts of the cytoplasmic matrix depended on the state of assembly of flagella or method of isolation of the matrix, we analyzed proteins extracted from steady-state flagella by ultrasound. The sedimentation profile of 35S-labeled proteins of this extract included three peaks, two of which had an apparent sedimentation coefficient of 17 S and 11 S, respectively (Fig. 2a). The 17S peak was formed by a group of 13 polypeptides (Fig. 2b) that was indistinguishable from that shown in Fig. 1b for the apparent molecular weight and molar ratio of its components. It represented ≈7% of the proteins resolved in the gradient and 2% of the flagellar protein mass.
The 11S peak shown in Fig. 2a included complexes of inner dynein arm subunits that were identified by immunoprecipitations with antibodies specific for p28 or caltractin/centrin, as reported before (6) (data not shown). The inner dynein arm subunits actin, p28, and caltractin/centrin are indicated by dots in Fig. 2b. These subunits were absent from extracts of the cytoplasmic matrix prepared by nonionic detergent (Fig. 1b). Therefore, they were released from the axoneme by the ultrasound. They provided an internal reference for this type of extract of the cytoplasmic matrix (see experiments shown in Fig. 5).
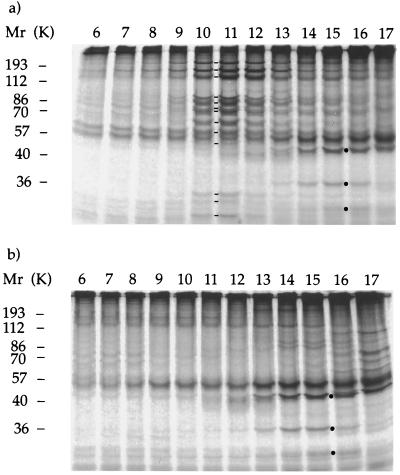
Figure 5.
The 17S complex requires the activity of KHP1 to be present in flagella. (a and b) Electrophoretograms of proteins contained in sucrose gradient fractions 6–17 resolving proteins extracted from fla10 flagella by ultrasound. Proteins from fla10 cells exposed at 21°C (a) and 32°C (b). Molecular weight standards are indicated on the left. Lines between lanes 10 and 11 of a indicate components 1–13 of the 17S complex. Dots between lanes 15 and 16 indicate inner dynein arm subunits actin, p28, and caltractin/centrin, respectively.
The 17S complex also was present in the matrix from flagella of wild-type cells that were exposed at 32°C for 1 h (data not shown).
Part of the 17S Complex Is Associated with p28-Containing Inner Dynein Arms.
The inner dynein arm subunit p28 is not one of the 13 subunits of the 17S complex but it could be associated with the 17S complex at a low molar ratio.
To determine whether p28 cosedimented with the 17S complex we performed a Western blot analysis of the same fractions shown in Fig. 1b by antibodies specific for p28. p28 formed a shallow peak of sedimentation with a maximum included in fractions 8–10 and apparent sedimentation coefficient of 18 S (Fig. 3a). p28 relative to the 17S complex was in a molar ratio 1:2 and 1:10 in fraction 8 and 10, respectively, as measured by the PhosphorImager.
Figure 3.
Western blots by antibodies specific for p28. (a) Gradient fractions resolving proteins of the cytoplasmic matrix from regenerated flagella. The polypeptide content of these fractions is that shown in Fig. 1b. (b) Gradient fractions resolving inner and outer dynein arms that were extracted from regenerated axonemes by 0.6 M NaCl and 1 mM ATP-Mg (18). This extract was prepared from axonemes of the same flagella that provided the cytoplasmic matrix. Furthermore, it was brought to the same ionic and detergent concentrations of the cytoplasmic matrix.
An analysis of the sedimentation behavior of p28-containing inner dynein arms that were extracted from the axonemes by 0.6 M NaCl and 1 mM ATP-Mg showed that these complexes formed a sharp peak of sedimentation with a maximum included in fractions 15–16 and an apparent sedimentation coefficient of 11 S (Fig. 3b) as observed before (21). Therefore, p28 from the matrix cosedimented with part of the 17S complex, whereas p28-containing complexes from the axoneme did not. This difference in sedimentation suggested that part of p28 may be carried by part of the 17S complex within the cytoplasmic matrix.
To confirm this hypothesis we exposed either steady-state or regenerating flagella to ultrasound, isolated the 17S complex, and subjected these protein fractions to column chromatography on DEAE-Sepharose. We analyzed the polypetides eluted from the column by gel electrophoresis and Western blots. Both protein fractions generated chromatograms that were similar (data not shown). The elution profile of the 17S complex (Fig. 4a) was coincident with the elution profile of p28 (Fig. 4b). Both profiles had a maximum eluted by 0.30 M NaCl and 0.01 M Hepes (pH 6.8). Under identical conditions of chromatography p28-containing 11S inner dynein arms formed a different elution profile (Fig. 4c). The maximum of this profile was eluted by 0.25 M NaCl and 0.01 M Hepes (pH 6.8).
Figure 4.
p28-containing inner dynein arms are associated with part of the 17S complex in the cytoplasmic matrix. (a) Electrophoretograms of subunits of the 17S complex contained in chromatographic fractions that were eluted from a 0.3 ml DEAE-Sepharose (fast flow; Pharmacia) column. Elution was performed by a 4 ml 0.03–0.4 M NaCl gradient in 0.01 M Hepes (pH 6.8). Molecular weight standards are indicated on the left. Lines between lanes 24 and 25 indicate the presence of components 1–13 of the 17S complex. (b and c) Western blots of polypeptides by antibodies specific for p28. (b) Western blot of fractions that are shown in a. (c) Western blot of 11S inner dynein arms that were subjected to DEAE-Sepharose column chromatography as described in a. (d) Electrophoretograms of polypeptides that were precipitated from a sucrose gradient fraction containing the 17S complex. Lane 1 (indicated as −) shows polypetides precipitated by the protein A-agarose beads in the absence of antibodies. Lane 2 (indicated as +) shows polypetides precipitated by the protein A-agarose beads in the presence of antibodies specific for p28. Dynein heavy chains (HC), actin, and p28 that are present in the immunoprecipitate are indicated on the right.
The immunoprecipitation of p28 from sucrose gradient fractions containing the 17S complex of steady-state or regenerating flagella showed that p28 was present in these fractions as part of inner dynein arms. p28 was immunoprecipitated with actin and inner dynein arm heavy chains (Fig. 4c).
The 17S Protein Complex Requires the Activity of KHP1 to be Present in Flagella.
To determine whether the 17S complex requires the activity of KHP1 to be present in the cytoplasmic matrix of steady state flagella, we analyzed extracts from fla10 flagella obtained by ultrasound after exposure of cells at permissive (21°C) or nonpermissive temperature (32°C) for 1 h. Following sucrose gradient sedimentation of the two extracts we resolved polypeptide components of the fractions by PAGE (Fig. 5). All components of the 17S complex were present in the extract prepared from fla10 cells exposed at permissive temperature (indicated by lines between lanes 10 and 11, Fig. 5a). In contrast, they were undetectable from the extract prepared from fla10 cells exposed at restrictive temperature (Fig. 5b). Furthermore, actin, p28, and caltractin/centrin (indicated by dots between lanes 15 and 16, Fig. 5) were present in both extracts in similar amounts. Subunits 1–7 of the 17S complex, all subunits that could be identified by their apparent molecular weight, were absent from the low density parts of the sucrose gradients resolving these two extracts (not shown).
Subunits 1, 2, 4–6, 8, and 10 could be identified in two-dimensional maps of flagellar polypeptides from fla10 cells exposed for 1 h at permissive temperature. In contrast, they were undetectable in two-dimensional maps of flagellar polypeptides from fla10 cells exposed for 1 h at restrictive temperature (data not shown).
Results of these experiments indicated that the activity of KHP1 was required for the presence of the 17S complex in both matrix and flagellum at steady state. Furthermore, subunits of the 17S complex were not dissociated from each other and did not become associated to the axoneme in fla10 flagella of cells that were exposed at restrictive temperature. Therefore, the 17S complex at restrictive temperature was transported back from flagella to the cell body of fla10 by a mechanism that is independent from the KHP1 activity.
The 17S Complex Turns Over More Rapidly than Dynein Subunits in the Axoneme.
The 17S complex could shuttle between cell body and flagella of wild-type cells and turn over more frequently than axonemal components. To provide evidence supporting the second hypothesis we determined whether pulse-labeling of the 17S complex subunits is faster than that of axonemal dynein arm subunits in steady-state flagella.
We analyzed the cytoplasmic matrix that was extracted from flagella by ultrasound after exposure of cells to [35S]H2SO4 for 30 min. Following sucrose gradient sedimentation of extracted proteins we resolved polypeptide components of sucrose fractions by PAGE (Fig. 6). Components 1–5, 7, 8, 10, and 11 of the 17S complex were pulse-labeled (indicated by lines between lanes 10 and 11, Fig. 6). In contrast, labeling of axonemal inner dynein arm subunits actin, p28, and caltractin/centrin was not evident as that shown in fractions 15 of Figs. 2b and 5.
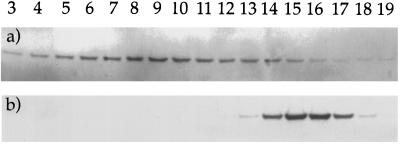
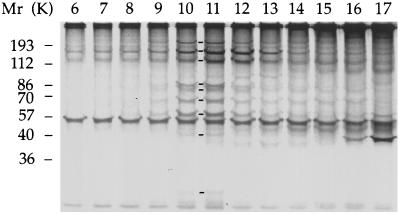
Figure 6.
Subunits of the 17S complex turn over in the cytoplasmic matrix more rapidly than inner dynein arm subunits actin, p28, and caltractin/centrin in the axonemes. Wild-type cells were cultured in medium containing reduced amount of sulfates and pulse labeled with [35S]H2SO4 for 30 min at 21°C. Matrix proteins were isolated by ultrasound. Electrophoretogram of pulse labeled proteins contained in sucrose gradient fractions 6–17. Molecular weight standards are indicated on the left. Lines between lanes 10 and 11 indicate the presence of components 1–5, 7, 8, 10, and 11 of the 17S complex.
Outer and inner dynein arm subunits extracted from the axonemes of pulse-labeled cells by 0.6 M NaCl and 1 mM ATP-Mg were resolved by sedimentation in a sucrose gradient and identified by their sedimentation behavior and electrophoretic mobility. They had a specific radioactivity 15–37 times lower than that of 17S complex subunits (data not shown).
DISCUSSION
The 13 polypeptides referred to as the 17S complex behave as a group in vivo and in vitro. As a group, they became undetectable in fla10 flagella at restrictive temperature. As a complex, they were isolated by two independent methods of extraction, followed by successive sedimentation in sucrose gradient and chromatography on a DEAE-Sepharose column.
The 17S complex is a component of the matrix of Chlamydomonas flagella and not a complex derived from the cell body or the axoneme. It represented 7–11% of protein extracts including the cytoplasmic matrix independently from both, method of extraction, and state of assembly of flagella. It was barely detectable in axonemes that were isolated under conditions preserving the axonemal structure (unpublished results).
The presence of the 17S complex in protein fractions including the cytoplasmic matrix was observed independently from the molecular composition of these fractions. Extracts obtained by exposing regenerated flagella to a nonionic detergent included cytoplasmic matrix and integral membrane proteins, whereas extracts obtained by exposing steady-state flagella to ultrasound included cytoplasmic matrix proteins and inner dynein arms that preferentially were disassembled from the axonemes.
The amount of the 17S complex in regenerating flagella is approximately two times higher than the amount of the 17S complex in steady-state flagella. This may due to a higher concentration of proteins in the cytoplasmic matrix of regenerating flagella. Higher concentration of matrix proteins and 17S complex may be needed for the assembly of flagellar structures. On the other hand the basal level of the 17S complex concentration in steady-state flagella may be required for the maintenance of flagellar structures.
Part of the 17S complex was bound to part of p28 in extracts of the cytoplasmic matrix that were prepared by two independent methods. The 17S complex functioned as a carrier of p28 by an interaction that probably was generated in vivo. That interaction survived under conditions adopted for both sedimentation in sucrose gradient and column chromatography on DEAE-Sepharose.
p28 is associated with the 17S complex as part of dyneins that contain inner dynein arm heavy chains and actin. It was immunoprecipitated together with inner dynein arm heavy chains and actin from sucrose gradient fractions containing both p28 and 17S complex. The association between p28-containing dyneins and the 17S complex resulted in the formation of a set of complexes sedimenting with a coefficient higher than 17S, as shown in Fig. 3a.
p28-containing dyneins bound to the 17S complex were distinguished from p28-containing inner dynein arms extracted from the axoneme by their sedimentation and chromatographic properties. They probably are precursors of inner dynein arms that are transported along flagella together with the 17S complex before being assembled to their final site of activity. Furthermore, they may not be the only precursors of axonemal components that are bound to the 17S complex because the majority of the 17S complex is not associated to p28.
The interaction between precursors of axonemal components and the 17S complex may involve the activity of chaperones (22). A monoclonal antibody that identified Hsp70 in Chlamydomonas flagella (20) bound to component 6 of the 17S complex (unpublished results). The presence of Hsp70 was observed before in the distal part of Chlamydomonas flagella by immunofluorescence microscopy (20).
The 17S complex and its subunits require the activity of KHP1 to be present in flagella. They were absent from flagellar extracts of fla10 cells exposed for 1 h at restrictive temperature, whereas axonemal inner dynein arms remained present. Furthermore, subunits of the 17S complex did not bind to the axoneme as a result of the inactivation of KHP1. Therefore, the 17S complex was transported from flagella to the cell body during the exposure at restrictive temperature. This retrograde motion is independent from the KHP1 activity and could be part of the shuttling that the 17S complex normally does between the cell body and flagella.
The last hypothesis is consistent with results of pulse-labeled experiments. The majority of the 17S complex subunits turned over with those pooled in the cell body more rapidly then axonemal dynein arms. The absence of pulse labeling of components 6, 9, 12, and 13 of the 17S complex may be indicative of lack of an exchange of these subunits with those located in the cell body. In this case the 17S complex may be formed by two modules, one shuttling between cell body and flagella and the other resident of the cytoplasmic matrix of flagella.
KHP1 is not a subunit of the 17S complex because it is present in fla10 flagella after 1-h exposure of fla10 cells at restrictive temperature (14), whereas the 17S complex is absent. Furthermore, KHP1 remained bound to outer doublet microtubules following the solubilization of flagellar membrane and required the exposure to ATP-Mg to be dissociated from the axoneme (12). In contrast, the 17S complex is soluble under conditions that solubilize the flagellar membrane.
These observations are consistent with the result of Western blot analyses of the 17S complex that we performed by an antibody specific for KHP1 (12) (kindly provided by John Hall, The Rockefeller University, New York). The antibodies specific for KHP1 did not bind to subunits of the 17S complex but did bind to an axonemal subunit of 90,000 apparent molecular weight (unpublished results).
KHP1 also is required for bidirectional movement of particles within flagella (14) and intraflagellar transport of a 16S protein complex, as reported recently in abstract form (23). This evidence is consistent with the hypothesis that the intraflagellar particles are formed by protein complexes (23). The 17S complex could be one of them.
In summary, our initial search for additional components of the machinery transporting the inner dynein arm subunit p28 along Chlamydomonas axonemes led to the identification of a previously undetected “17S” protein complex that requires the activity of KHP1 to be present in the flagellar space. The 17S complex is a major component of the cytoplasmic matrix independently from the state of assembly of flagella, is composed of multiple subunits, and in part is associated with p28-containing inner dynein arms. The 17S complex may have different functions in the flagellar matrix. It may stabilize, transport and target precursors of inner dynein arms and other axonemal components. The study of other temperature-dependent assembly mutants (24, 25) representing at least six different loci may reveal different aspects of the 17S complex function.
Acknowledgments
We are grateful to Scott Henderson and Serafin Piñol-Roma (Mount Sinai School of Medicine, New York) and Michel LeDizet (University of California, San Francisco) for a critical reading of this manuscript. This work was supported by a grant from the National Institutes of Health (GM-44467).
References
- 1.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. Molecular Biology of the Cell. New York: Garland; 1994. [Google Scholar]
- 2.Smith E F, Sale W S. Science. 1992;257:1557–1559. doi: 10.1126/science.1387971. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein M, Rosenbaum J L. Trends Cell Biol. 1994;4:236–240. doi: 10.1016/0962-8924(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 4.Lewin R A. J Gen Microbiol. 1954;11:358–363. doi: 10.1099/00221287-11-3-358. [DOI] [PubMed] [Google Scholar]
- 5.Luck D J L. J Cell Biol. 1984;98:789–794. doi: 10.1083/jcb.98.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeDizet M, Piperno G. Mol Biol Cell. 1995;6:697–711. doi: 10.1091/mbc.6.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brokaw C J, Kamiya R. Cell Motil Cytoskel. 1987;8:68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- 8.Piperno G, Ramanis Z. J Cell Biol. 1991;112:701–709. doi: 10.1083/jcb.112.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King S J, Inwood W B, O’Toole E T, Power J, Dutcher S K. J Cell Biol. 1994;126:1255–66. doi: 10.1083/jcb.126.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piperno G, Mead K, Henderson S. J Cell Biol. 1996;133:371–379. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell D R, Kang Y. J Cell Biol. 1991;113:835–42. doi: 10.1083/jcb.113.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walther Z, Vashishtha M, Hall J L. J Cell Biol. 1994;126:175–188. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vashishtha M, Walther Z, Hall J L. J Cell Sci. 1996;109:541–549. doi: 10.1242/jcs.109.3.541. [DOI] [PubMed] [Google Scholar]
- 14.Kozminski G K, Beech P L, Rosenbaum J L. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozminski K G, Johnson K A, Forscher P, Rosenbaum J L. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum J L, Moulder J E, Ringo D L. J Cell Biol. 1969;41:600–19. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luck D J L, Piperno G, Ramanis Z, Huang B. Proc Natl Acad Sci USA. 1977;74:3456–3460. doi: 10.1073/pnas.74.8.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piperno G, Luck D J L. J Biol Chem. 1979;254:3084–3090. [PubMed] [Google Scholar]
- 19.Piperno G. Cilia and Flagella. Orlando, FL: Academic; 1995. pp. 107–112. [Google Scholar]
- 20.Bloch M A, Johnson K A. J Cell Sci. 1995;108:3541–3545. doi: 10.1242/jcs.108.11.3541. [DOI] [PubMed] [Google Scholar]
- 21.Piperno G, Ramanis Z, Smith E F, Sale W S. J Cell Biol. 1990;110:379–389. doi: 10.1083/jcb.110.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartl F U. Nature (London) 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 23.Cole D G, Rosenbaum J L. Mol Biol Cell. 1996;7:47a. (abstr.). [Google Scholar]
- 24.Huang B, Rifkin M R, Luck D J L. J Cell Biol. 1977;72:67–85. doi: 10.1083/jcb.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams G M W, Huang B, Luck D J L. Genetics. 1982;100:579–586. doi: 10.1093/genetics/100.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]