Abstract
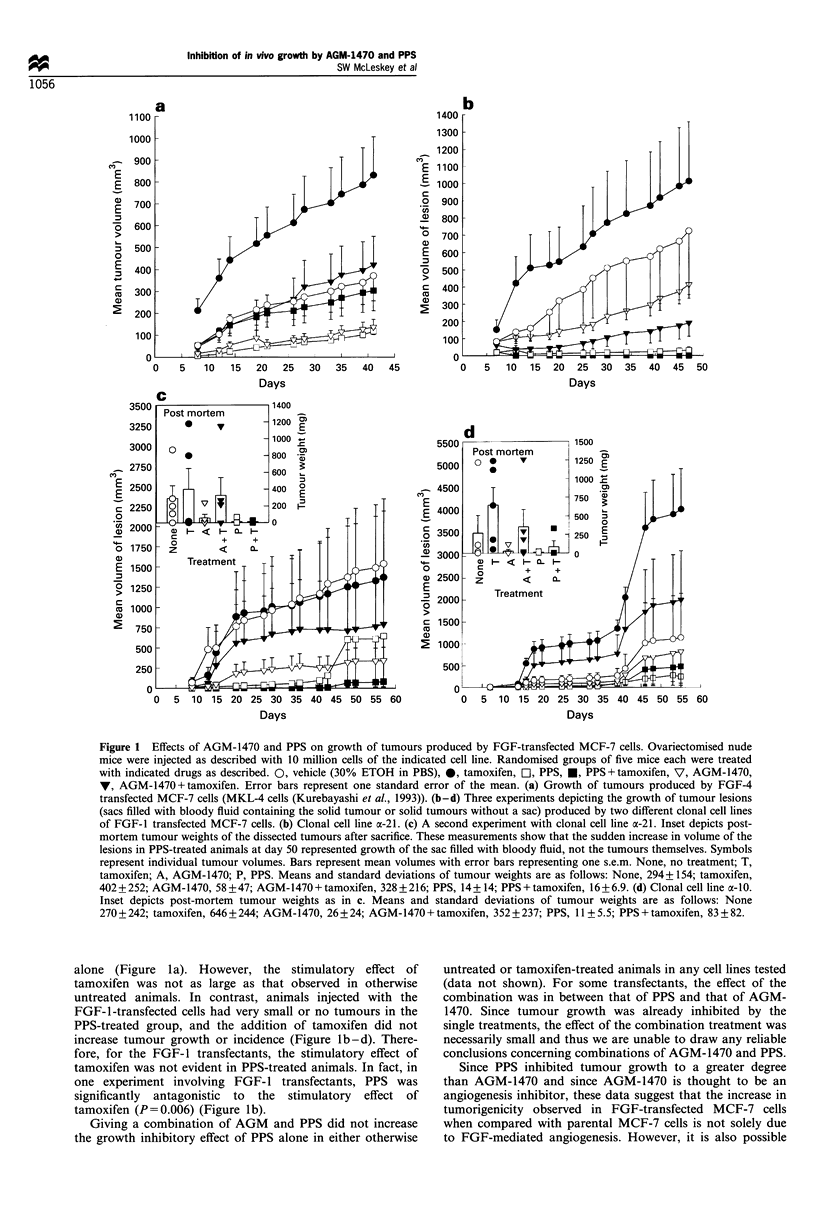
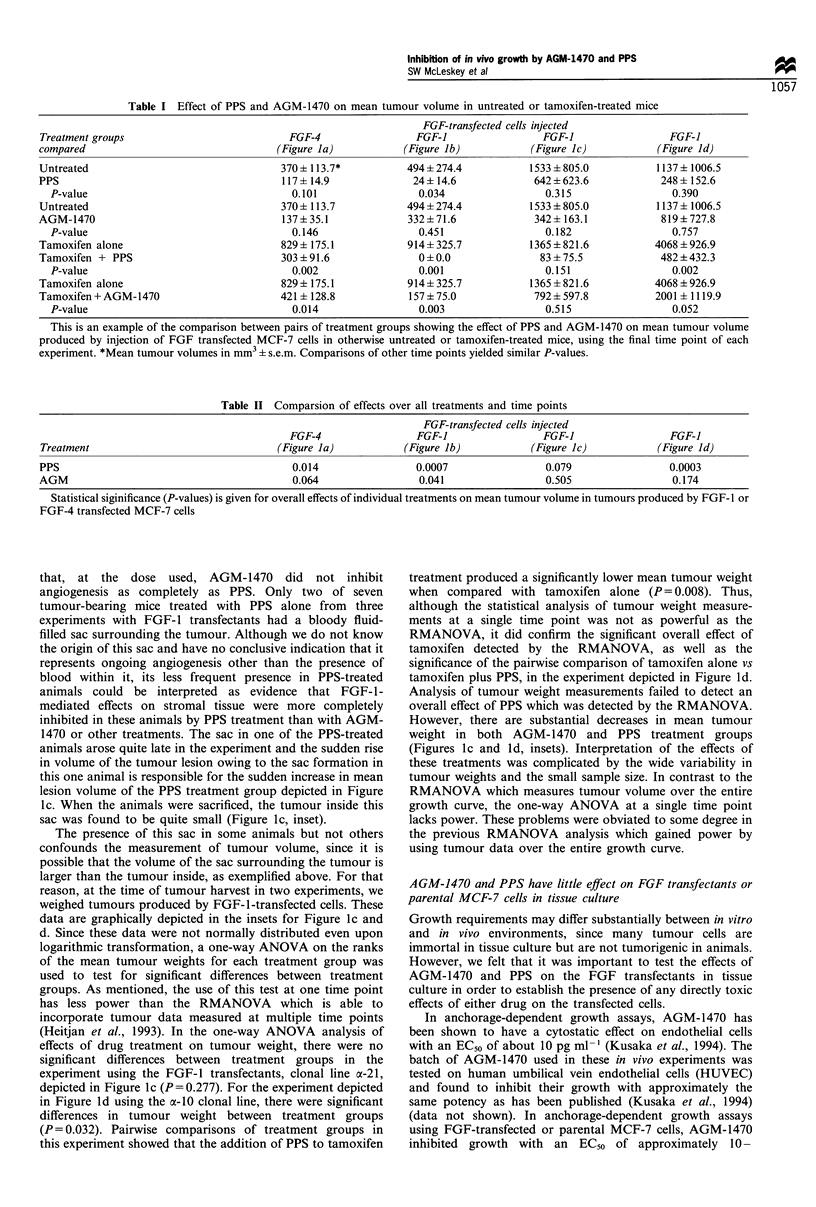
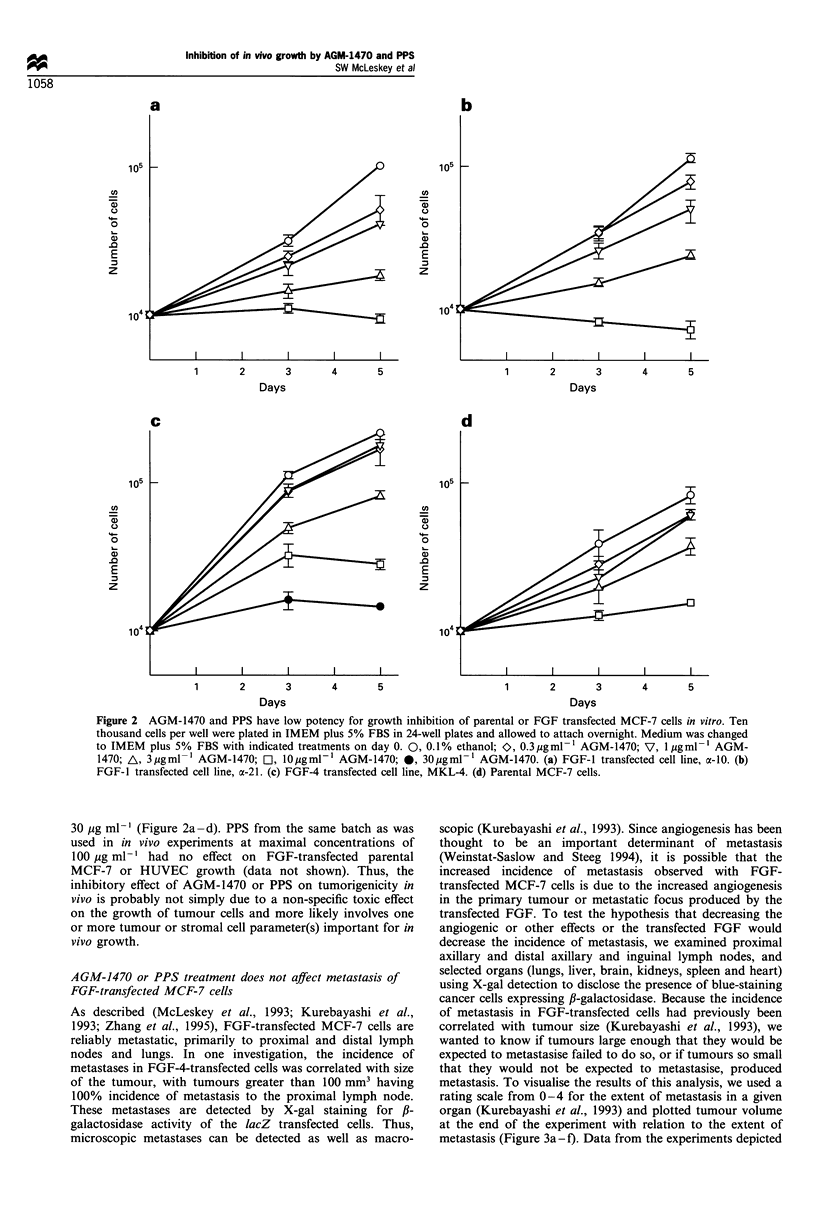
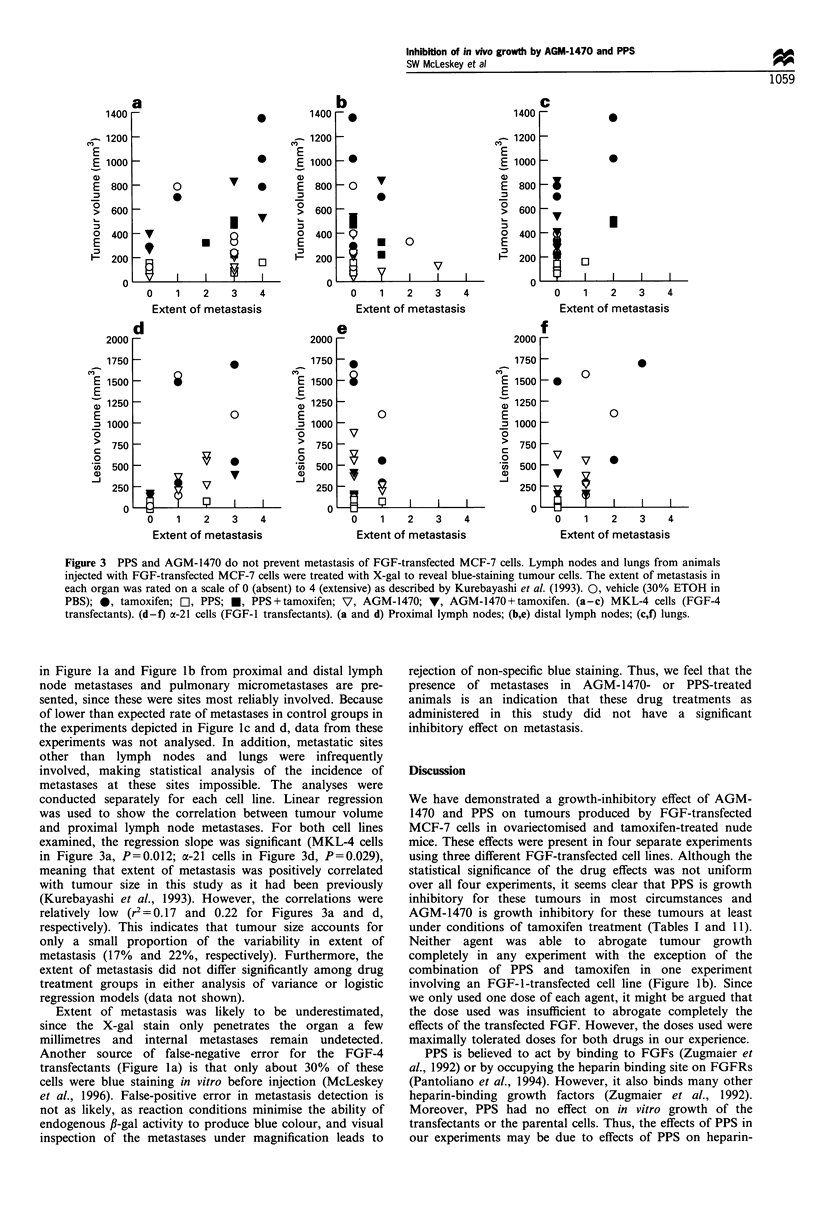
Previously, we described FGF-1- or FGF-4-transfected MCF-7 breast carcinoma cells which are tumorigenic and metastatic in untreated or tamoxifen-treated ovariectomised nude mice. In this study, we have assessed the effects of AGM-1470, an antiangiogenic agent, and pentosan polysulphate (PPS), an agent that abrogates the effects of FGFs, on tumour growth and metastasis produced by these FGF-transfected MCF-7 cells. Untreated or tamoxifen-treated ovariectomised mice were injected with FGF-transfected cells, treated with AGM-1470 or PPS, and tumour growth and metastasis analysed. The sensitivity of FGF-transfected and parental MCF-7 cells to AGM-1470 or PPS was also determined in vitro. Both AGM-1470 and PPS inhibited tumour growth in otherwise untreated or tamoxifen-treated mice injected with either FGF- or FGF-4-transfected MCF-7 cells. This effect was more reliably seen in tamoxifen-treated animals. AGM-1470 was about 10(5) times less potent in inhibiting the anchorage-dependent growth of parental MCF-7 or FGF-transfected MCF-7 cells than in inhibiting the growth of human umbilical vein endothelial cells. PPS did not affect the in vitro growth of the transfectants or parental cells. Thus, the growth-inhibitory effect on tumours was in excess of the effect of either drug on the same cells in tissue culture, implying that stromal elements are important determinants of the effects of these drugs. There was a positive correlation between tumour size and the extent of proximal lymph node metastasis. However, neither drug had a significant effect on the extent of metastasis to proximal or distal lymph nodes or lungs. AGM-1470 or PPS may be helpful in cases of breast carcinoma in which angiogenesis is due to expression of FGFs by the tumour cells and may be more effective when combined with tamoxifen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe J., Zhou W., Takuwa N., Taguchi J., Kurokawa K., Kumada M., Takuwa Y. A fumagillin derivative angiogenesis inhibitor, AGM-1470, inhibits activation of cyclin-dependent kinases and phosphorylation of retinoblastoma gene product but not protein tyrosyl phosphorylation or protooncogene expression in vascular endothelial cells. Cancer Res. 1994 Jul 1;54(13):3407–3412. [PubMed] [Google Scholar]
- Antoine N., Greimers R., De Roanne C., Kusaka M., Heinen E., Simar L. J., Castronovo V. AGM-1470, a potent angiogenesis inhibitor, prevents the entry of normal but not transformed endothelial cells into the G1 phase of the cell cycle. Cancer Res. 1994 Apr 15;54(8):2073–2076. [PubMed] [Google Scholar]
- Arteaga C. L., Carty-Dugger T., Moses H. L., Hurd S. D., Pietenpol J. A. Transforming growth factor beta 1 can induce estrogen-independent tumorigenicity of human breast cancer cells in athymic mice. Cell Growth Differ. 1993 Mar;4(3):193–201. [PubMed] [Google Scholar]
- Arteaga C. L., Tandon A. K., Von Hoff D. D., Osborne C. K. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988 Jul 15;48(14):3898–3904. [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Belford D. A., Hendry I. A., Parish C. R. Investigation of the ability of several naturally occurring and synthetic polyanions to bind to and potentiate the biological activity of acidic fibroblast growth factor. J Cell Physiol. 1993 Oct;157(1):184–189. doi: 10.1002/jcp.1041570124. [DOI] [PubMed] [Google Scholar]
- Brem S., Tsanaclis A. M., Gately S., Gross J. L., Herblin W. F. Immunolocalization of basic fibroblast growth factor to the microvasculature of human brain tumors. Cancer. 1992 Dec 1;70(11):2673–2680. doi: 10.1002/1097-0142(19921201)70:11<2673::aid-cncr2820701118>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Mehlman T., Marshak D. R., Fraser B. A., Maciag T. Structural evidence that endothelial cell growth factor beta is the precursor of both endothelial cell growth factor alpha and acidic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7216–7220. doi: 10.1073/pnas.83.19.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butta A., MacLennan K., Flanders K. C., Sacks N. P., Smith I., McKinna A., Dowsett M., Wakefield L. M., Sporn M. B., Baum M. Induction of transforming growth factor beta 1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. 1992 Aug 1;52(15):4261–4264. [PubMed] [Google Scholar]
- Dalal B. I., Keown P. A., Greenberg A. H. Immunocytochemical localization of secreted transforming growth factor-beta 1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am J Pathol. 1993 Aug;143(2):381–389. [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Forough R., Xi Z., MacPhee M., Friedman S., Engleka K. A., Sayers T., Wiltrout R. H., Maciag T. Differential transforming abilities of non-secreted and secreted forms of human fibroblast growth factor-1. J Biol Chem. 1993 Feb 5;268(4):2960–2968. [PubMed] [Google Scholar]
- Gagliardi A., Collins D. C. Inhibition of angiogenesis by antiestrogens. Cancer Res. 1993 Feb 1;53(3):533–535. [PubMed] [Google Scholar]
- Gajdusek C. M., Luo Z., Mayberg M. R. Basic fibroblast growth factor and transforming growth factor beta-1: synergistic mediators of angiogenesis in vitro. J Cell Physiol. 1993 Oct;157(1):133–144. doi: 10.1002/jcp.1041570118. [DOI] [PubMed] [Google Scholar]
- Gomm J. J., Smith J., Ryall G. K., Baillie R., Turnbull L., Coombes R. C. Localization of basic fibroblast growth factor and transforming growth factor beta 1 in the human mammary gland. Cancer Res. 1991 Sep 1;51(17):4685–4692. [PubMed] [Google Scholar]
- Halaban R. Growth regulation in normal and malignant melanocytes. Recent Results Cancer Res. 1993;128:133–150. doi: 10.1007/978-3-642-84881-0_10. [DOI] [PubMed] [Google Scholar]
- Haran E. F., Maretzek A. F., Goldberg I., Horowitz A., Degani H. Tamoxifen enhances cell death in implanted MCF7 breast cancer by inhibiting endothelium growth. Cancer Res. 1994 Nov 1;54(21):5511–5514. [PubMed] [Google Scholar]
- Heitjan D. F., Manni A., Santen R. J. Statistical analysis of in vivo tumor growth experiments. Cancer Res. 1993 Dec 15;53(24):6042–6050. [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Kato T., Sato K., Kakinuma H., Matsuda Y. Enhanced suppression of tumor growth by combination of angiogenesis inhibitor O-(chloroacetyl-carbamoyl)fumagillol (TNP-470) and cytotoxic agents in mice. Cancer Res. 1994 Oct 1;54(19):5143–5147. [PubMed] [Google Scholar]
- Kurebayashi J, Kurosumi M, Dickson RB, Sonoo H. Angiogenesis Inhibitor O-(Chloroacetyl-carbamoyl) fumagillol (TNP-470) Inhibits Tumor Angiogenesis, Growth and Spontaneous Metastasis of MKL-4 Human Breast Cancer Cells in Female Athymic Nude Mice. Breast Cancer. 1994 Dec 30;1(2):109–115. doi: 10.1007/BF02967040. [DOI] [PubMed] [Google Scholar]
- Kusaka M., Sudo K., Fujita T., Marui S., Itoh F., Ingber D., Folkman J. Potent anti-angiogenic action of AGM-1470: comparison to the fumagillin parent. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1070–1076. doi: 10.1016/0006-291x(91)91529-l. [DOI] [PubMed] [Google Scholar]
- Kusaka M., Sudo K., Matsutani E., Kozai Y., Marui S., Fujita T., Ingber D., Folkman J. Cytostatic inhibition of endothelial cell growth by the angiogenesis inhibitor TNP-470 (AGM-1470). Br J Cancer. 1994 Feb;69(2):212–216. doi: 10.1038/bjc.1994.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeskey S. W., Kurebayashi J., Honig S. F., Zwiebel J., Lippman M. E., Dickson R. B., Kern F. G. Fibroblast growth factor 4 transfection of MCF-7 cells produces cell lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude mice. Cancer Res. 1993 May 1;53(9):2168–2177. [PubMed] [Google Scholar]
- Mori S., Ueda T., Kuratsu S., Hosono N., Izawa K., Uchida A. Suppression of pulmonary metastasis by angiogenesis inhibitor TNP-470 in murine osteosarcoma. Int J Cancer. 1995 Mar 29;61(1):148–152. doi: 10.1002/ijc.2910610125. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Hobbs K., Clark G. M. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985 Feb;45(2):584–590. [PubMed] [Google Scholar]
- Pantoliano M. W., Horlick R. A., Springer B. A., Van Dyk D. E., Tobery T., Wetmore D. R., Lear J. D., Nahapetian A. T., Bradley J. D., Sisk W. P. Multivalent ligand-receptor binding interactions in the fibroblast growth factor system produce a cooperative growth factor and heparin mechanism for receptor dimerization. Biochemistry. 1994 Aug 30;33(34):10229–10248. doi: 10.1021/bi00200a003. [DOI] [PubMed] [Google Scholar]
- Placidi L., Cretton-Scott E., de Sousa G., Rahmani R., Placidi M., Sommadossi J. P. Disposition and metabolism of the angiogenic moderator O-(chloroacetyl-carbamoyl) fumagillol (TNP-470; AGM-1470) in human hepatocytes and tissue microsomes. Cancer Res. 1995 Jul 15;55(14):3036–3042. [PubMed] [Google Scholar]
- Smith J., Yelland A., Baillie R., Coombes R. C. Acidic and basic fibroblast growth factors in human breast tissue. Eur J Cancer. 1994;30A(4):496–503. doi: 10.1016/0959-8049(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Sun L., Wu G., Willson J. K., Zborowska E., Yang J., Rajkarunanayake I., Wang J., Gentry L. E., Wang X. F., Brattain M. G. Expression of transforming growth factor beta type II receptor leads to reduced malignancy in human breast cancer MCF-7 cells. J Biol Chem. 1994 Oct 21;269(42):26449–26455. [PubMed] [Google Scholar]
- Walker R. A., Dearing S. J. Transforming growth factor beta 1 in ductal carcinoma in situ and invasive carcinomas of the breast. Eur J Cancer. 1992;28(2-3):641–644. doi: 10.1016/s0959-8049(05)80116-9. [DOI] [PubMed] [Google Scholar]
- Weinstat-Saslow D., Steeg P. S. Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. FASEB J. 1994 Apr 1;8(6):401–407. doi: 10.1096/fasebj.8.6.7513289. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Fabra A., Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F., Saya H., Bruner J. M., Morrison R. S. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):484–488. doi: 10.1073/pnas.91.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka M., Yamamoto T., Masaki T., Ikeyama S., Sudo K., Fujita T. Inhibition of tumor growth and metastasis of rodent tumors by the angiogenesis inhibitor O-(chloroacetyl-carbamoyl)fumagillol (TNP-470; AGM-1470). Cancer Res. 1993 Sep 15;53(18):4262–4267. [PubMed] [Google Scholar]
- Yan G., Fukabori Y., McBride G., Nikolaropolous S., McKeehan W. L. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol. 1993 Aug;13(8):4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase T., Tamura M., Fujita K., Kodama S., Tanaka K. Inhibitory effect of angiogenesis inhibitor TNP-470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res. 1993 Jun 1;53(11):2566–2570. [PubMed] [Google Scholar]
- Zugmaier G., Lippman M. E., Wellstein A. Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals. J Natl Cancer Inst. 1992 Nov 18;84(22):1716–1724. doi: 10.1093/jnci/84.22.1716. [DOI] [PubMed] [Google Scholar]



