Abstract
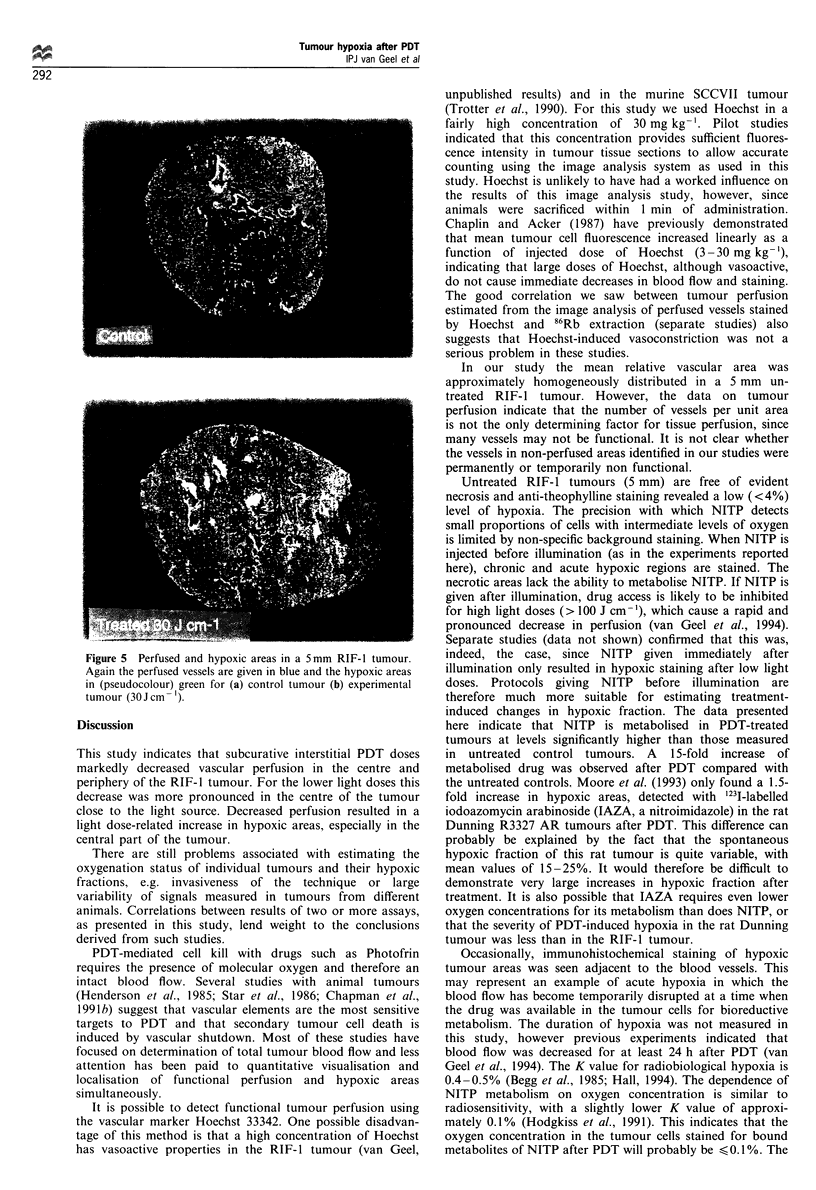
The influence of photodynamic therapy (PDT) on vascular perfusion and the development of hypoxia was investigated in the murine RIF-1 tumour. Image analysis was used to quantify changes in perfusion and hypoxia at 5 min after interstitial Photofrin-mediated PDT. The fluorescent stain Hoechst 33342 was used as an in vivo marker of functional vascular perfusion and the antibody anti-collagen type IV as a marker of the tumour vasculature. The percentage of total tumour vasculature that was perfused decreased to less than 30% of control values after PDT. For the lower light doses this decrease was more pronounced in the centre of the tumour. The observed reduction in vascular perfusion showed a good linear correlation (r = 0.98) with previously published tumour perfusion data obtained with the 86Rb extraction technique. The image analysis technique provides extra information concerning the localisation of (non)-perfused vessels. To detect hypoxic tumour areas in vivo, an immunohistochemical method was used employing NITP [7-(4'-(2-nitroimidazol-1-yl)-butyl)-theophylline]. A large increase in hypoxic areas was found for PDT-treated tumours. More than half the total tumour area was hypoxic after PDT, compared with < 4% for control tumours. Our studies illustrate the potential of image analysis systems for monitoring the functional consequences of PDT-mediated vascular damage early after treatment. This provides direct confirmation that the perfusion changes lead to tissue hypoxia, which has implications for the combined treatment of PDT with bioreductive drugs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P., Michielsen C., Oppelaar H., van Zandwijk N., Stewart F. A. Enhancement of interstitial photodynamic therapy by mitomycin C and EO9 in a mouse tumour model. Int J Cancer. 1994 Mar 15;56(6):880–885. doi: 10.1002/ijc.2910560621. [DOI] [PubMed] [Google Scholar]
- Baas P., Oppelaar H., Stavenuiter M., van Zandwijk N., Stewart F. A. Interaction of the bioreductive drug SR 4233 and photodynamic therapy using photofrin in a mouse tumor model. Int J Radiat Oncol Biol Phys. 1993 Oct 20;27(3):665–670. doi: 10.1016/0360-3016(93)90394-b. [DOI] [PubMed] [Google Scholar]
- Begg A. C., Hodgkiss R. J., McNally N. J., Middleton R. W., Stratford M. R., Terry N. H. Fluorescent markers of hypoxic cells: a comparison of two compounds on three cell lines. Br J Radiol. 1985 Jul;58(691):645–654. doi: 10.1259/0007-1285-58-691-645. [DOI] [PubMed] [Google Scholar]
- Bernsen H. J., Rijken P. F., Oostendorp T., van der Kogel A. J. Vascularity and perfusion of human gliomas xenografted in the athymic nude mouse. Br J Cancer. 1995 Apr;71(4):721–726. doi: 10.1038/bjc.1995.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. C., Adams G. E., Pearson J. K., Sansom J. M., Stratford I. J., Bedwell J., Bown S. G., MacRobert A. J., Phillips D. Increasing the effect of photodynamic therapy on the RIF-1 murine sarcoma, using the bioreductive drugs RSU1069 and RB6145. Br J Cancer. 1992 Dec;66(6):1070–1076. doi: 10.1038/bjc.1992.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987 Jan 15;47(2):597–601. [PubMed] [Google Scholar]
- Chapman J. D., Stobbe C. C., Arnfield M. R., Santus R., Lee J., McPhee M. S. Oxygen dependency of tumor cell killing in vitro by light-activated Photofrin II. Radiat Res. 1991 Apr;126(1):73–79. [PubMed] [Google Scholar]
- Coco-Martin J. M., Brunink F., van der Velden-de Groot T. A., Beuvery E. C. Analysis of glycoforms present in two mouse IgG2a monoclonal antibody preparations. J Immunol Methods. 1992 Nov 5;155(2):241–248. doi: 10.1016/0022-1759(92)90291-z. [DOI] [PubMed] [Google Scholar]
- Foster T. H., Murant R. S., Bryant R. G., Knox R. S., Gibson S. L., Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res. 1991 Jun;126(3):296–303. doi: 10.2307/3577919. [DOI] [PubMed] [Google Scholar]
- Gonzalez S., Arnfield M. R., Meeker B. E., Tulip J., Lakey W. H., Chapman J. D., McPhee M. S. Treatment of Dunning R3327-AT rat prostate tumors with photodynamic therapy in combination with misonidazole. Cancer Res. 1986 Jun;46(6):2858–2862. [PubMed] [Google Scholar]
- Henderson B. W., Fingar V. H. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987 Jun 15;47(12):3110–3114. [PubMed] [Google Scholar]
- Henderson B. W., Waldow S. M., Mang T. S., Potter W. R., Malone P. B., Dougherty T. J. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985 Feb;45(2):572–576. [PubMed] [Google Scholar]
- Hirsch B. D., Walz N. C., Meeker B. E., Arnfield M. R., Tulip J., McPhee M. S., Chapman J. D. Photodynamic therapy-induced hypoxia in rat tumors and normal tissues. Photochem Photobiol. 1987 Nov;46(5):847–852. doi: 10.1111/j.1751-1097.1987.tb04858.x. [DOI] [PubMed] [Google Scholar]
- Hodgkiss R. J., Jones G., Long A., Parrick J., Smith K. A., Stratford M. R., Wilson G. D. Flow cytometric evaluation of hypoxic cells in solid experimental tumours using fluorescence immunodetection. Br J Cancer. 1991 Jan;63(1):119–125. doi: 10.1038/bjc.1991.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. J. Unusual oxygen concentration dependence of toxicity of SR-4233, a hypoxic cell toxin. Cancer Res. 1993 Sep 1;53(17):3992–3997. [PubMed] [Google Scholar]
- Marshall R. S., Rauth A. M. Modification of the cytotoxic activity of mitomycin C by oxygen and ascorbic acid in Chinese hamster ovary cells and a repair-deficient mutant. Cancer Res. 1986 Jun;46(6):2709–2713. [PubMed] [Google Scholar]
- Moore R. B., Chapman J. D., Mercer J. R., Mannan R. H., Wiebe L. I., McEwan A. J., McPhee M. S. Measurement of PDT-induced hypoxia in Dunning prostate tumors by iodine-123-iodoazomycin arabinoside. J Nucl Med. 1993 Mar;34(3):405–411. [PubMed] [Google Scholar]
- Mulcahy R. T. Effect of oxygen on misonidazole chemosensitization and cytotoxicity in vitro. Cancer Res. 1984 Oct;44(10):4409–4413. [PubMed] [Google Scholar]
- Star W. M., Marijnissen H. P., van den Berg-Blok A. E., Versteeg J. A., Franken K. A., Reinhold H. S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986 May;46(5):2532–2540. [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Y. C., Rauth A. M. Oxygen tension, cellular respiration, and redox state as variables influencing the cytotoxicity of the radiosensitizer misonidazole. Radiat Res. 1982 Jul;91(1):104–123. [PubMed] [Google Scholar]
- Trotter M. J., Chaplin D. J., Durand R. E., Olive P. L. The use of fluorescent probes to identify regions of transient perfusion in murine tumors. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):931–934. doi: 10.1016/0360-3016(89)90889-4. [DOI] [PubMed] [Google Scholar]
- Trotter M. J., Olive P. L., Chaplin D. J. Effect of vascular marker Hoechst 33342 on tumour perfusion and cardiovascular function in the mouse. Br J Cancer. 1990 Dec;62(6):903–908. doi: 10.1038/bjc.1990.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- van Geel I. P., Oppelaar H., Oussoren Y. G., Schuitmaker J. J., Stewart F. A. Mechanisms for optimising photodynamic therapy: second-generation photosensitisers in combination with mitomycin C. Br J Cancer. 1995 Aug;72(2):344–350. doi: 10.1038/bjc.1995.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geel I. P., Oppelaar H., Oussoren Y. G., Stewart F. A. Changes in perfusion of mouse tumours after photodynamic therapy. Int J Cancer. 1994 Jan 15;56(2):224–228. doi: 10.1002/ijc.2910560214. [DOI] [PubMed] [Google Scholar]
- van Geel I. P., Oppelaar H., Oussoren Y. G., van der Valk M. A., Stewart F. A. Photosensitizing efficacy of MTHPC-PDT compared to photofrin-PDT in the RIF1 mouse tumour and normal skin. Int J Cancer. 1995 Jan 27;60(3):388–394. doi: 10.1002/ijc.2910600320. [DOI] [PubMed] [Google Scholar]