Abstract
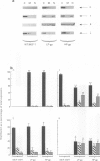
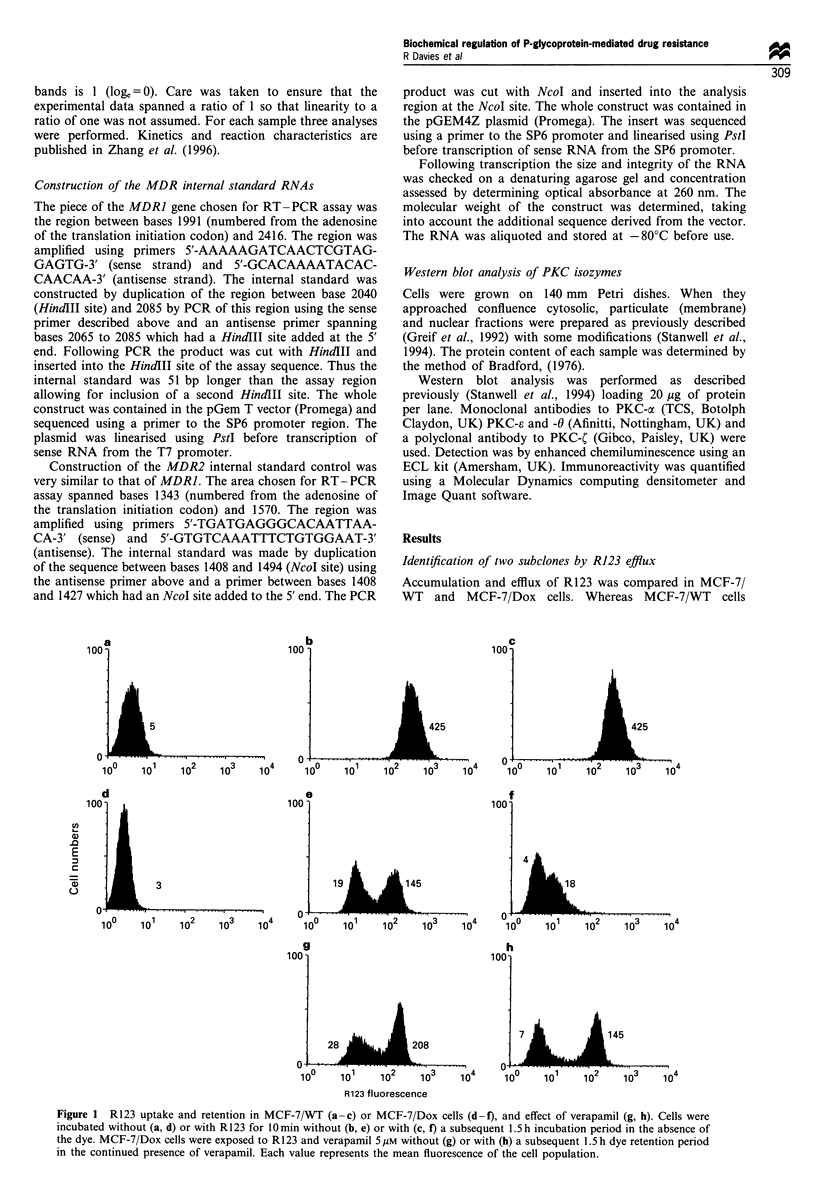
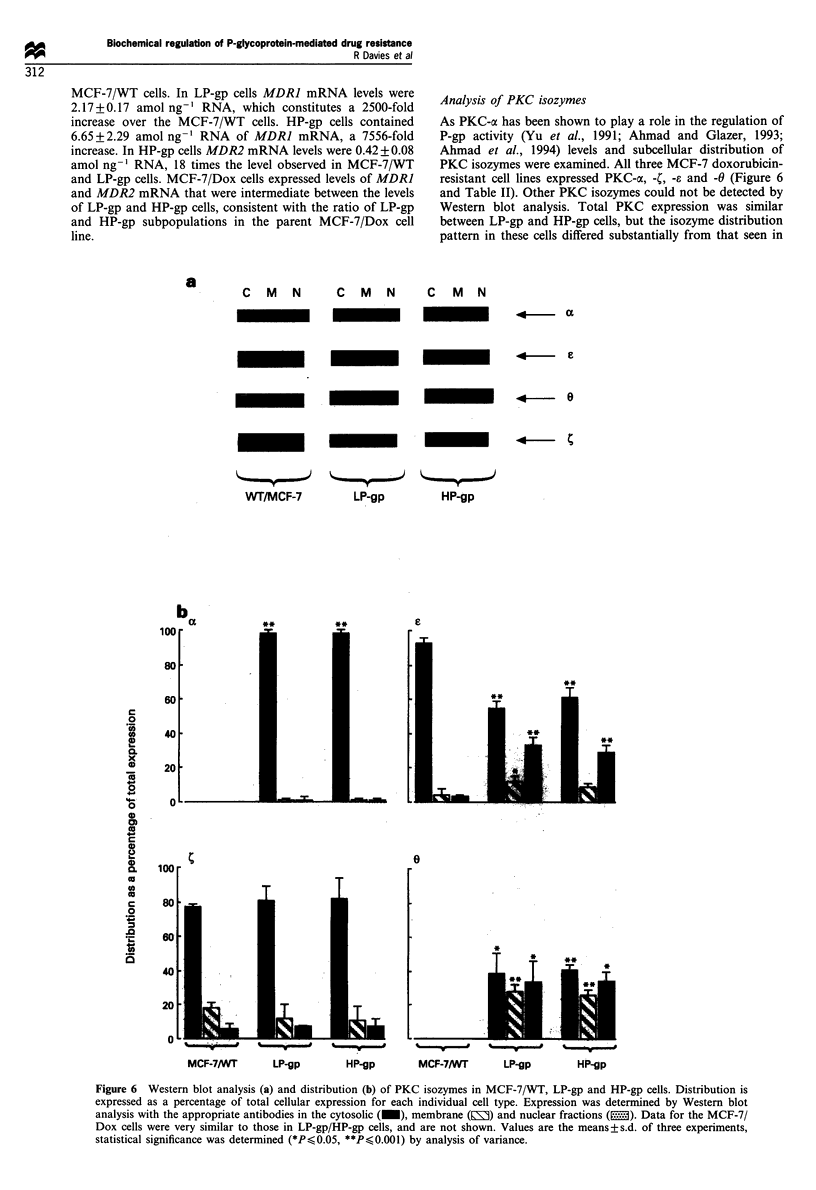
The MCF-7 doxorubicin-resistant cell line MCF-7/Dox has been used extensively for studies of the multidrug resistance phenomenon. Using fluorescence-activated cell sorting (FACS), these cells were separated into two populations on the basis of rhodamine 123 (R123) accumulation. We designated these as low P-glycoprotein (LP-gp) and high P-gp (HP-gp) cells on the basis of their P-gp content. Using the reverse transcriptase polymerase chain reaction technique controlled by homologous internal standards, we analysed levels of MDR1 and MDR2 mRNA in each cell type. LP-gp and HP-gp cells had MDR1 mRNA levels of 2.17 +/- 0.17 and 6.65 +/- 2.29 amol ng-1 total RNA respectively, compared with 0.00088 +/- 0.00005 amol ng-1 in wild-type MCF-7 cells (MCF-7/WT). MCF-7/WT cells additionally contained 0.023 +/- 0.016 amol ng-1 of MDR2 mRNA, which was unchanged in LP-gp cells, but lower than in HP-gp cells, which contained 0.42 +/- 0.08 amol ng-1. Both LP-gp and HP-gp cells contained increased copies of the MDR1 gene. However, the degree of gene amplification did not correlate with the changes in MDR1 mRNA levels, indicating further regulatory levels of gene expression. The level of P-gp detected by MRK 16 correlated with R123 accumulation. HP-gp cells expressed a 10-fold higher level of P-gp1 than LP-gp cells. However, there was only a 3-fold increase in MDR1 mRNA level in HP-gp cells compared with LP-gp cells. These data suggest that some regulation of P-gp1 expression also occurred at the post-translational level. Phosphorylation of P-gp by protein kinase C (PKC)-alpha is necessary for its activity. Our analysis of PKC-alpha, 0 and epsilon isozyme levels, and subcellular distribution, shows a co-regulation of expression with P-gp, suggesting a necessary role for PKC in P-gp regulation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Glazer R. I. Expression of the antisense cDNA for protein kinase C alpha attenuates resistance in doxorubicin-resistant MCF-7 breast carcinoma cells. Mol Pharmacol. 1993 Jun;43(6):858–862. [PubMed] [Google Scholar]
- Ahmad S., Safa A. R., Glazer R. I. Modulation of P-glycoprotein by protein kinase C alpha in a baculovirus expression system. Biochemistry. 1994 Aug 30;33(34):10313–10318. doi: 10.1021/bi00200a011. [DOI] [PubMed] [Google Scholar]
- Bates S. E., Lee J. S., Dickstein B., Spolyar M., Fojo A. T. Differential modulation of P-glycoprotein transport by protein kinase inhibition. Biochemistry. 1993 Sep 7;32(35):9156–9164. doi: 10.1021/bi00086a022. [DOI] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Thorgeirsson S. S., Silverman J. A. Cloning and regulation of the rat mdr2 gene. Nucleic Acids Res. 1993 Aug 11;21(16):3885–3891. doi: 10.1093/nar/21.16.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. C., McAvoy E. M., Jacobs J. W., Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem. 1990 May 5;265(13):7679–7686. [PubMed] [Google Scholar]
- Chambers T. C., Zheng B., Kuo J. F. Regulation by phorbol ester and protein kinase C inhibitors, and by a protein phosphatase inhibitor (okadaic acid), of P-glycoprotein phosphorylation and relationship to drug accumulation in multidrug-resistant human KB cells. Mol Pharmacol. 1992 Jun;41(6):1008–1015. [PubMed] [Google Scholar]
- Chan H. S., Bradley G., Thorner P., Haddad G., Gallie B. L., Ling V. A sensitive method for immunocytochemical detection of P-glycoprotein in multidrug-resistant human ovarian carcinoma cell lines. Lab Invest. 1988 Dec;59(6):870–875. [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Activation of MDR1 (P-glycoprotein) gene expression in human cells by protein kinase C agonists. Oncol Res. 1992;4(7):281–290. [PubMed] [Google Scholar]
- Chin J. E., Soffir R., Noonan K. E., Choi K., Roninson I. B. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol. 1989 Sep;9(9):3808–3820. doi: 10.1128/mcb.9.9.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Frommel T. O., Stern R. K., Perez C. F., Kriegler M., Tsuruo T., Roninson I. B. Multidrug resistance after retroviral transfer of the human MDR1 gene correlates with P-glycoprotein density in the plasma membrane and is not affected by cytotoxic selection. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7386–7390. doi: 10.1073/pnas.88.16.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier S. J., Kane S. E., Willingham M. C., Cardarelli C. O., Pastan I., Gottesman M. M. Identification of residues in the first cytoplasmic loop of P-glycoprotein involved in the function of chimeric human MDR1-MDR2 transporters. J Biol Chem. 1992 Dec 15;267(35):25153–25159. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Kao-Shan C. S., Whang-Peng J., Rosen N., Israel M. A., Melera P. W., Cowan K. H., Goldsmith M. E. Isolation of amplified and overexpressed DNA sequences from adriamycin-resistant human breast cancer cells. Cancer Res. 1987 Oct 1;47(19):5141–5148. [PubMed] [Google Scholar]
- Fine R. L., Patel J., Chabner B. A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):582–586. doi: 10.1073/pnas.85.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futscher B. W., Blake L. L., Gerlach J. H., Grogan T. M., Dalton W. S. Quantitative polymerase chain reaction analysis of mdr1 mRNA in multiple myeloma cell lines and clinical specimens. Anal Biochem. 1993 Sep;213(2):414–421. doi: 10.1006/abio.1993.1440. [DOI] [PubMed] [Google Scholar]
- Gant T. W., Silverman J. A., Bisgaard H. C., Burt R. K., Marino P. A., Thorgeirsson S. S. Regulation of 2-acetylaminofluorene-and 3-methylcholanthrene--mediated induction of multidrug resistance and cytochrome P450IA gene family expression in primary hepatocyte cultures and rat liver. Mol Carcinog. 1991;4(6):499–509. doi: 10.1002/mc.2940040614. [DOI] [PubMed] [Google Scholar]
- Gant T. W., Silverman J. A., Thorgeirsson S. S. Regulation of P-glycoprotein gene expression in hepatocyte cultures and liver cell lines by a trans-acting transcriptional repressor. Nucleic Acids Res. 1992 Jun 11;20(11):2841–2846. doi: 10.1093/nar/20.11.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greif H., Ben-Chaim J., Shimon T., Bechor E., Eldar H., Livneh E. The protein kinase C-related PKC-L(eta) gene product is localized in the cell nucleus. Mol Cell Biol. 1992 Mar;12(3):1304–1311. doi: 10.1128/mcb.12.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kartner N., Shales M., Riordan J. R., Ling V. Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res. 1983 Sep;43(9):4413–4419. [PubMed] [Google Scholar]
- Kohno K., Tanimura H., Sato S., Nakayama Y., Makino Y., Wada M., Fojo A. T., Kuwano M. Cellular control of human multidrug resistance 1 (mdr-1) gene expression in absence and presence of gene amplification in human cancer cells. J Biol Chem. 1994 Aug 12;269(32):20503–20508. [PubMed] [Google Scholar]
- Lee C. H., Bradley G., Zhang J. T., Ling V. Differential expression of P-glycoprotein genes in primary rat hepatocyte culture. J Cell Physiol. 1993 Nov;157(2):392–402. doi: 10.1002/jcp.1041570223. [DOI] [PubMed] [Google Scholar]
- Ludescher C., Hilbe W., Eisterer W., Preuss E., Huber C., Gotwald M., Hofmann J., Thaler J. Activity of P-glycoprotein in B-cell chronic lymphocytic leukemia determined by a flow cytometric assay. J Natl Cancer Inst. 1993 Nov 3;85(21):1751–1758. doi: 10.1093/jnci/85.21.1751. [DOI] [PubMed] [Google Scholar]
- Ma L. D., Marquardt D., Takemoto L., Center M. S. Analysis of P-glycoprotein phosphorylation in HL60 cells isolated for resistance to vincristine. J Biol Chem. 1991 Mar 25;266(9):5593–5599. [PubMed] [Google Scholar]
- Madden M. J., Morrow C. S., Nakagawa M., Goldsmith M. E., Fairchild C. R., Cowan K. H. Identification of 5' and 3' sequences involved in the regulation of transcription of the human mdr1 gene in vivo. J Biol Chem. 1993 Apr 15;268(11):8290–8297. [PubMed] [Google Scholar]
- Molinari A., Cianfriglia M., Meschini S., Calcabrini A., Arancia G. P-glycoprotein expression in the Golgi apparatus of multidrug-resistant cells. Int J Cancer. 1994 Dec 15;59(6):789–795. doi: 10.1002/ijc.2910590614. [DOI] [PubMed] [Google Scholar]
- Morrow C. S., Chiu J., Cowan K. H. Posttranscriptional control of glutathione S-transferase pi gene expression in human breast cancer cells. J Biol Chem. 1992 May 25;267(15):10544–10550. [PubMed] [Google Scholar]
- Ng W. F., Sarangi F., Zastawny R. L., Veinot-Drebot L., Ling V. Identification of members of the P-glycoprotein multigene family. Mol Cell Biol. 1989 Mar;9(3):1224–1232. doi: 10.1128/mcb.9.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979 Dec 25;254(24):12701–12705. [PubMed] [Google Scholar]
- Schinkel A. H., Arceci R. J., Smit J. J., Wagenaar E., Baas F., Dollé M., Tsuruo T., Mechetner E. B., Roninson I. B., Borst P. Binding properties of monoclonal antibodies recognizing external epitopes of the human MDR1 P-glycoprotein. Int J Cancer. 1993 Sep 30;55(3):478–484. doi: 10.1002/ijc.2910550326. [DOI] [PubMed] [Google Scholar]
- Schinkel A. H., Roelofs E. M., Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Res. 1991 May 15;51(10):2628–2635. [PubMed] [Google Scholar]
- Shen D. W., Cardarelli C., Hwang J., Cornwell M., Richert N., Ishii S., Pastan I., Gottesman M. M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986 Jun 15;261(17):7762–7770. [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Slapak C. A., Kharbanda S., Saleem A., Kufe D. W. Defective translocation of protein kinase C in multidrug-resistant HL-60 cells confers a reversible loss of phorbol ester-induced monocytic differentiation. J Biol Chem. 1993 Jun 15;268(17):12267–12273. [PubMed] [Google Scholar]
- Stanwell C., Gescher A., Bradshaw T. D., Pettit G. R. The role of protein kinase C isoenzymes in the growth inhibition caused by bryostatin 1 in human A549 lung and MCF-7 breast carcinoma cells. Int J Cancer. 1994 Feb 15;56(4):585–592. doi: 10.1002/ijc.2910560420. [DOI] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Ahmad S., Aquino A., Fairchild C. R., Trepel J. B., Ohno S., Suzuki K., Tsuruo T., Cowan K. H., Glazer R. I. Transfection with protein kinase C alpha confers increased multidrug resistance to MCF-7 cells expressing P-glycoprotein. Cancer Commun. 1991 Jun;3(6):181–189. doi: 10.3727/095535491820873263. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]