Abstract
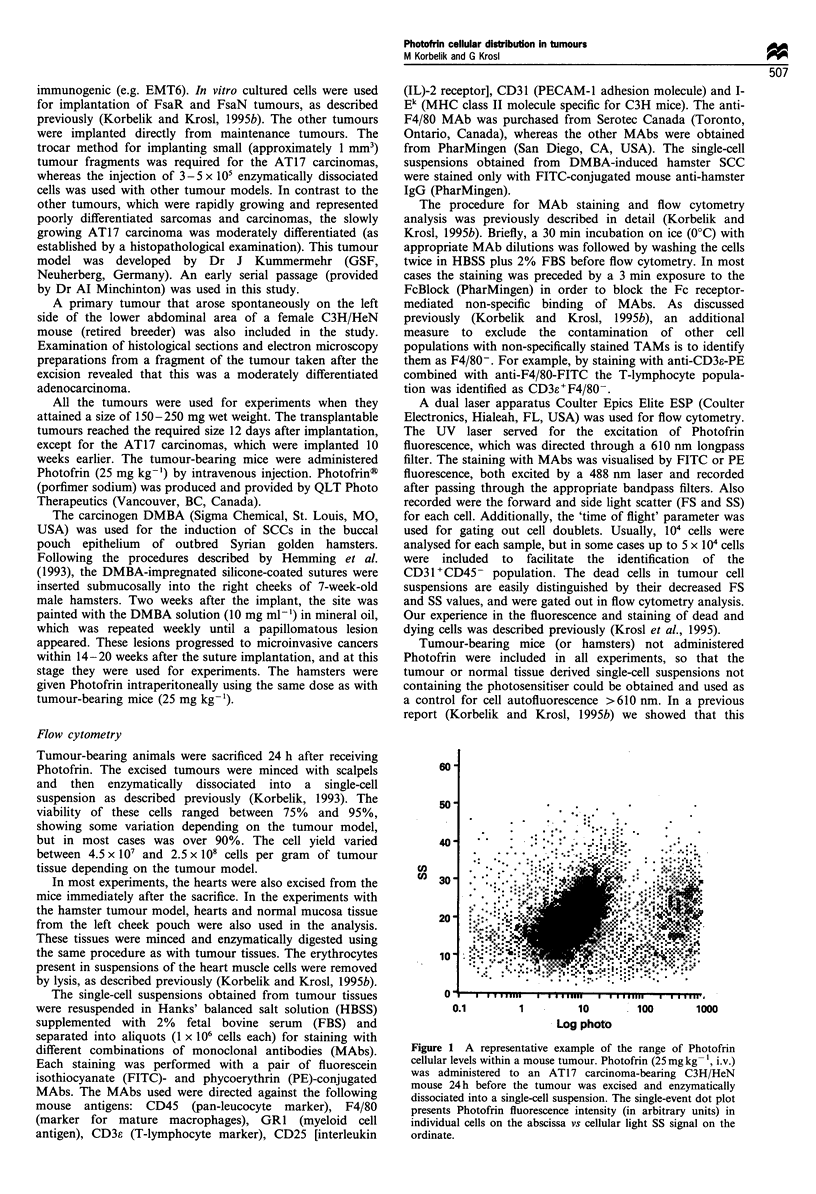
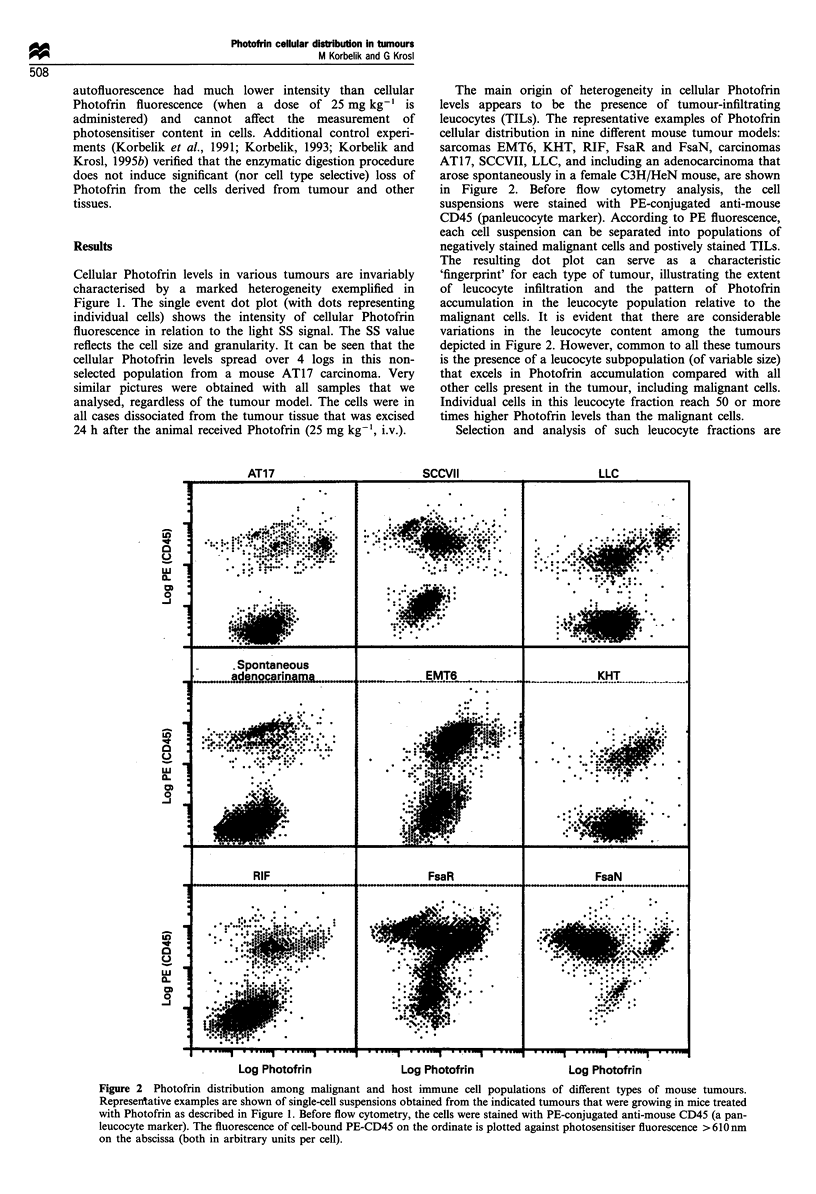
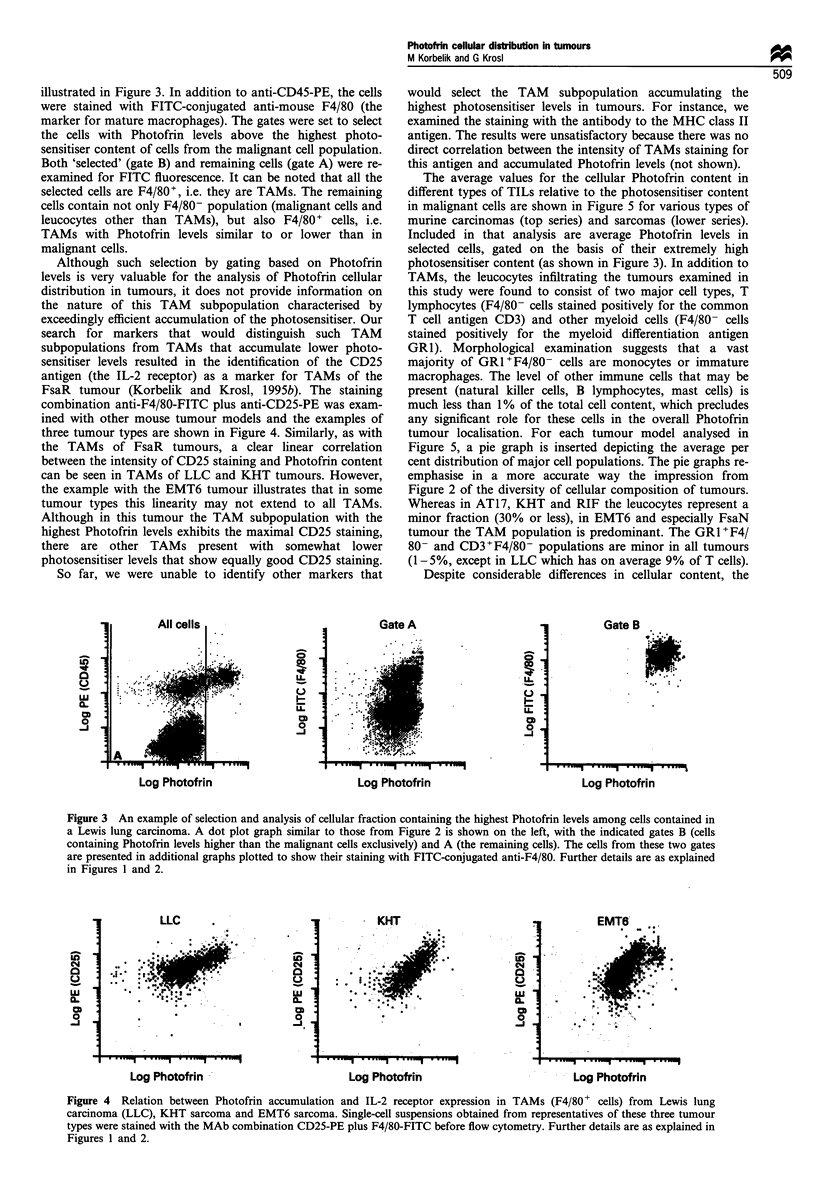
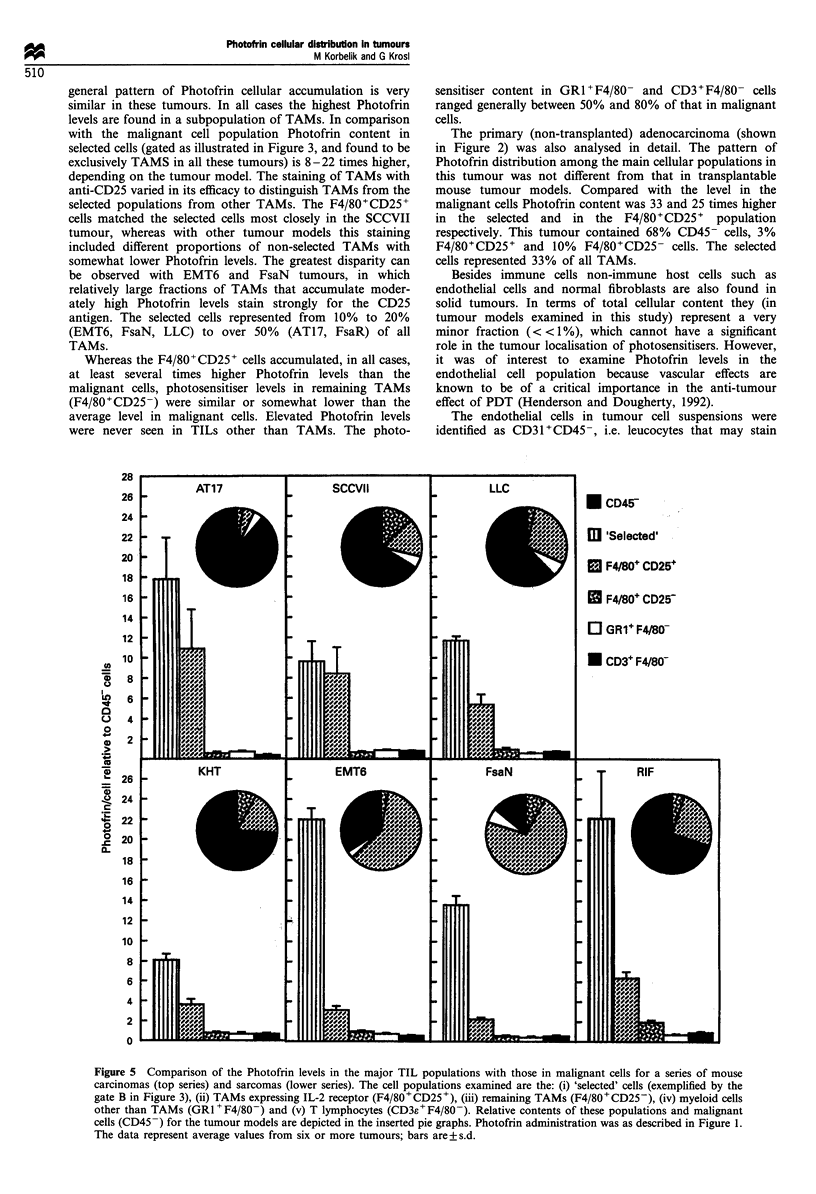
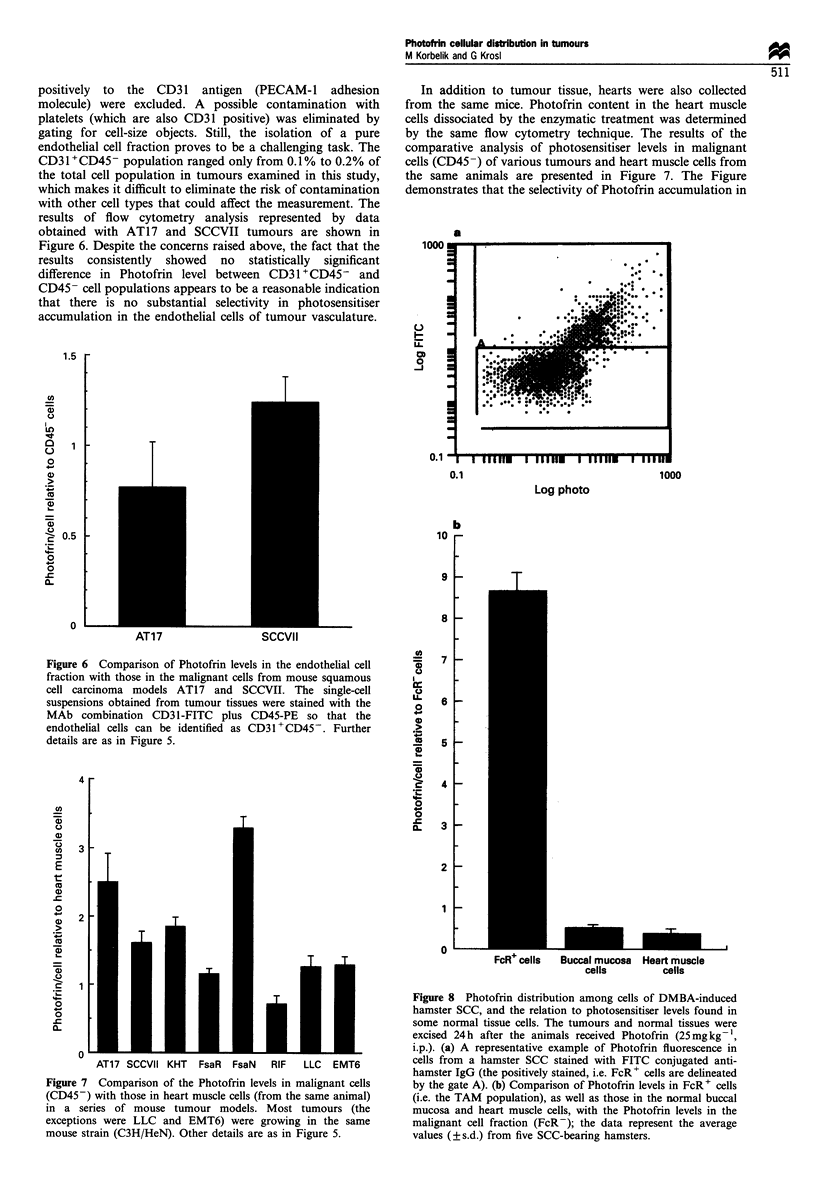
Photofrin accumulation in malignant and host cell populations of various tumours was studied by flow cytometry analysis of cells dissociated from the tumour tissue. The transplantable mouse tumour models included in this analysis were sarcomas EMT6, RIF, KHT and FsaN, Lewis lung carcinoma, SCCVII squamous cell carcinoma (SCC) and slowly growing moderately differentiated AT17 SCC. An example of spontaneous mouse adenocarcinoma was also examined. Staining with specific monoclonal antibodies was used to identify the various cell populations present in these tumours. The main characteristic of Photofrin cellular accumulation was a very high photosensitiser content found exclusively in a subpopulation of tumour-associated macrophages (TAMs). Photosensitiser levels similar to or lower than in malignant cells were observed in the remaining TAMs and other tumour-infiltrating host cells. Photofrin accumulation in malignant cells was not equal in all tumour models, but may have been affected by tumour blood perfusion/vascularisation. Results consistent with the above findings were obtained with SCC of buccal mucosa induced by 9,10-dimethyl-1,2-benzanthracene in Syrian hamsters. The TAM subpopulation that accumulates by far the highest cellular Photofrin levels in tumours is suggested to be responsible for the tumour-localised photosensitiser fluorescence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bucana C. D., Fabra A., Sanchez R., Fidler I. J. Different patterns of macrophage infiltration into allogeneic-murine and xenogeneic-human neoplasms growing in nude mice. Am J Pathol. 1992 Nov;141(5):1225–1236. [PMC free article] [PubMed] [Google Scholar]
- Chapman M. J. Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol. 1986;128:70–143. doi: 10.1016/0076-6879(86)28063-5. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Studies on the structure of porphyrins contained in Photofrin II. Photochem Photobiol. 1987 Nov;46(5):569–573. doi: 10.1111/j.1751-1097.1987.tb04815.x. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Rucker N., Murphree A. L. Differential cell photosensitivity following porphyrin photodynamic therapy. Cancer Res. 1988 Aug 15;48(16):4539–4542. [PubMed] [Google Scholar]
- Hemming A. W., Davis N. L., Dubois B., Quenville N. F., Finley R. J. Photodynamic therapy of squamous cell carcinoma. An evaluation of a new photosensitizing agent, benzoporphyrin derivative and new photoimmunoconjugate. Surg Oncol. 1993;2(3):187–196. doi: 10.1016/0960-7404(93)90006-k. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Dougherty T. J. How does photodynamic therapy work? Photochem Photobiol. 1992 Jan;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Fingar V. H. Oxygen limitation of direct tumor cell kill during photodynamic treatment of a murine tumor model. Photochem Photobiol. 1989 Mar;49(3):299–304. doi: 10.1111/j.1751-1097.1989.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Kallman R. F., Silini G., Van Putten L. M. Factors influencing the quantitative estimation of the in vivo survival of cells from solid tumors. J Natl Cancer Inst. 1967 Sep;39(3):539–549. [PubMed] [Google Scholar]
- Korbelik M. Distribution of disulfonated and tetrasulfonated aluminum phthalocyanine between malignant and host cell populations of a murine fibrosarcoma. J Photochem Photobiol B. 1993 Oct;20(2-3):173–181. doi: 10.1016/1011-1344(93)80148-3. [DOI] [PubMed] [Google Scholar]
- Korbelik M., Krosl G. Cellular levels of photosensitisers in tumours: the role of proximity to the blood supply. Br J Cancer. 1994 Oct;70(4):604–610. doi: 10.1038/bjc.1994.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbelik M., Krosl G., Olive P. L., Chaplin D. J. Distribution of Photofrin between tumour cells and tumour associated macrophages. Br J Cancer. 1991 Sep;64(3):508–512. doi: 10.1038/bjc.1991.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbelik M., Krosl G. Photofrin accumulation in malignant and host cell populations of a murine fibrosarcoma. Photochem Photobiol. 1995 Jul;62(1):162–168. doi: 10.1111/j.1751-1097.1995.tb05253.x. [DOI] [PubMed] [Google Scholar]
- Krosl G., Korbelik M., Dougherty G. J. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br J Cancer. 1995 Mar;71(3):549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Moan J., Farrants G., Danielsen H. E., Rimington C. Localization of potent photosensitizers in human tumor LOX by means of laser scanning microscopy. Cancer Lett. 1990 Sep;53(2-3):129–139. doi: 10.1016/0304-3835(90)90205-c. [DOI] [PubMed] [Google Scholar]
- Rockwell S. C., Kallman R. F., Fajardo L. F. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst. 1972 Sep;49(3):735–749. [PubMed] [Google Scholar]
- SALLEY J. J. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res. 1954 Apr;33(2):253–262. doi: 10.1177/00220345540330021201. [DOI] [PubMed] [Google Scholar]
- SUGIURA K., STOCK C. C. Studies in a tumor spectrum. III. The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Cancer Res. 1955 Jan;15(1):38–51. [PubMed] [Google Scholar]
- Suit H. D., Sedlacek R. S., Silver G., Dosoretz D. Pentobarbital anesthesia and the response of tumor and normal tissue in the C3Hf/sed mouse to radiation. Radiat Res. 1985 Oct;104(1):47–65. [PubMed] [Google Scholar]
- Svennevig J. L., Svaar H. Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer. 1979 Dec 15;24(6):754–758. doi: 10.1002/ijc.2910240609. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Volpe J. P., Hunter N., Basic I., Milas L. Metastatic properties of murine sarcomas and carcinomas. I. Positive correlation with lung colonization and lack of correlation with s.c. tumor take. Clin Exp Metastasis. 1985 Oct-Dec;3(4):281–294. doi: 10.1007/BF01585082. [DOI] [PubMed] [Google Scholar]


