Abstract
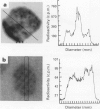
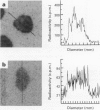
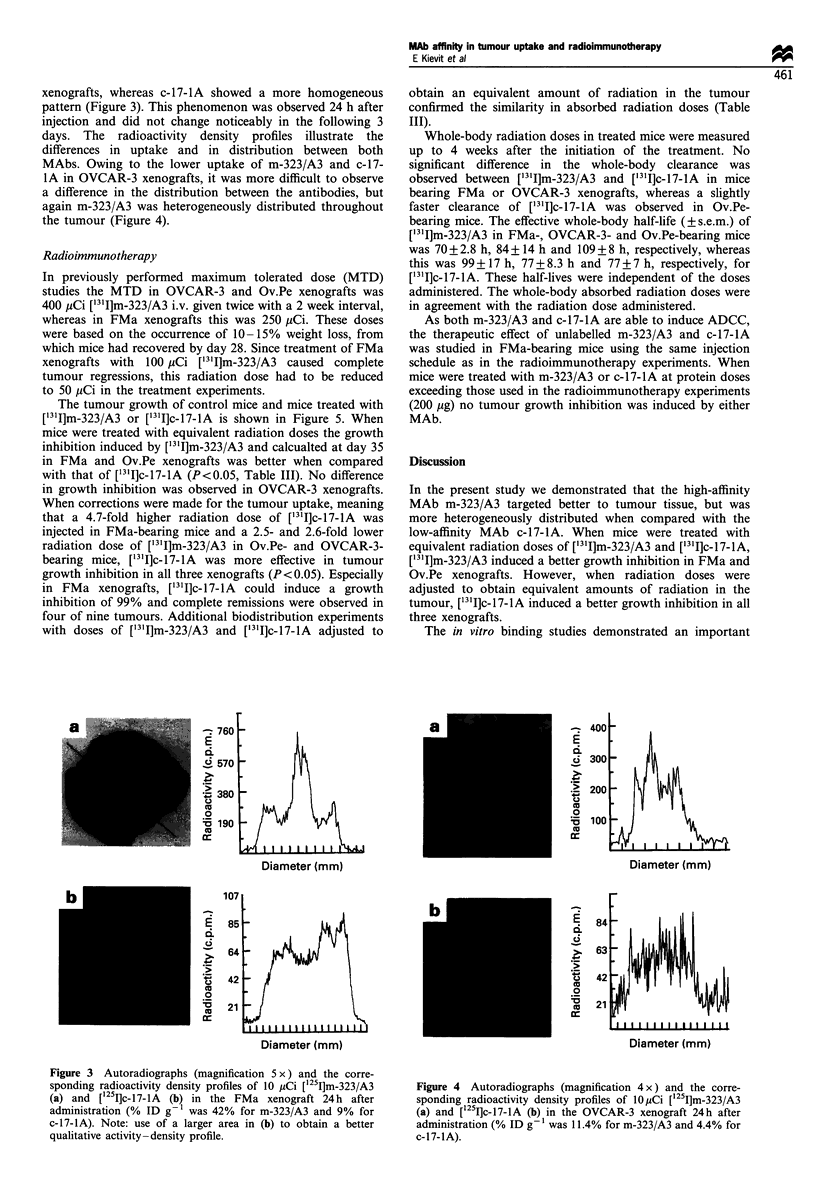
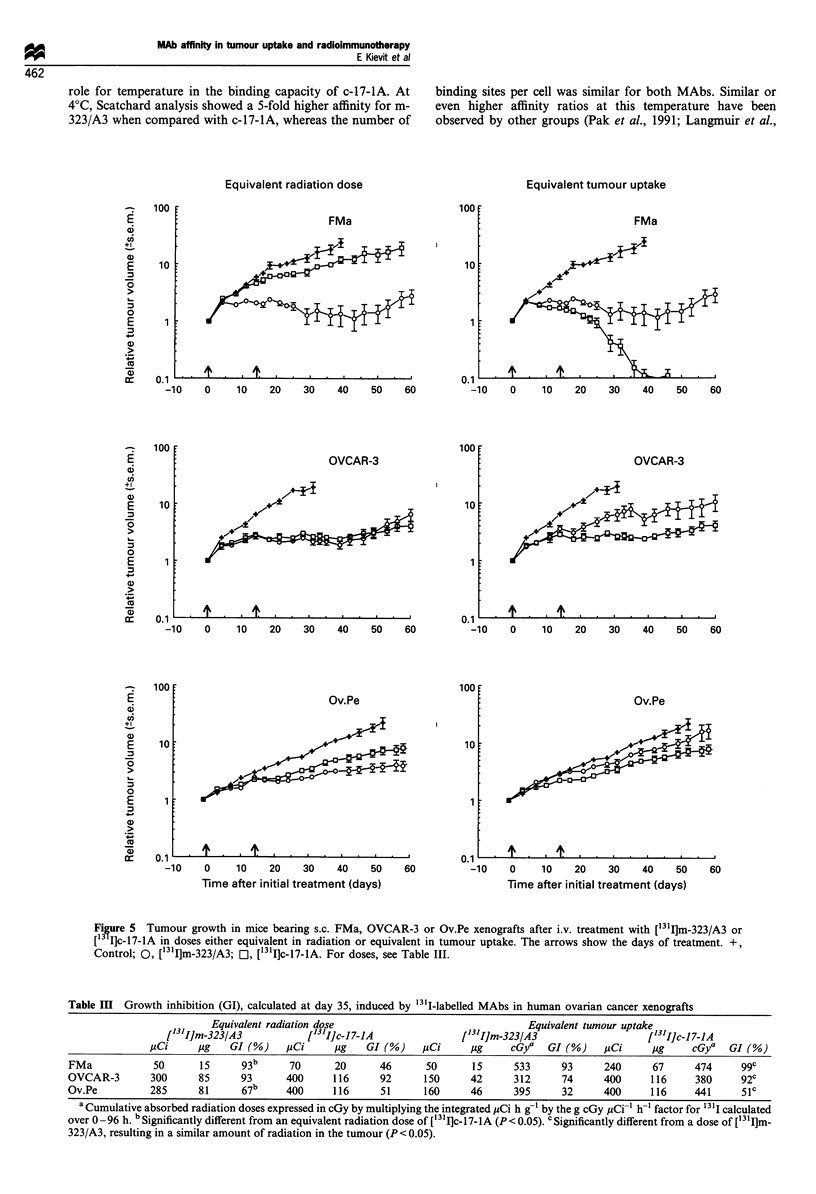
The low-affinity monoclonal antibody (MAb) chimeric 17-1A(c-17-1A) and the high-affinity MAb mouse 323/A3 (m-323/A3) were used to study the effect of the MAb affinity on the tumour uptake and efficacy of radioimmunotherapy in nude mice bearing subcutaneously the human ovarian cancer xenografts FMa, OVCAR-3 and Ov.Pe. Both MAbs are directed against the same pancarcinoma glycoprotein. In vitro, the number of binding sites on tumour cells at 4 degrees C was similar for both MAbs, but m-323/A3 had an approximately 5-fold higher affinity (1.3-3.0x10(9) M-1) than c-17-1A (3.0-5.4x10(8) M-1). This difference in affinity was more extreme at 37 degrees C, when no binding of c-17-1A could be observed. MAb m-323/A3 completely blocked binding of c-17-1A to tumour cells, whereas the reverse was not observed. Immunohistochemistry showed a similar but more intense staining pattern of m-323/A3 in human ovarian cancer xenografts than of c-17-1A. In vivo, the blood clearance in non-tumour-bearing nude mice was similar for both MAbs with terminal half-lives of 71.4 h for m-323/A3 and 62.7 h for c-17-1A. MAb m-323/A3 targeted better to tumour tissue, but was more heterogeneously distributed than c-17-1A. The cumulative absorbed radiation dose delivered by m-323/A3 to tumour tissue was 2.5- to 4.7-fold higher than that delivered by c-17-1A. When mice were treated with equivalent radiation doses of 131(I)m-323/A3 and 131(I)c-17-1A, based on a correction for the immunoreactivity of the radiolabelled MAbs, m-323/A3 induced a better growth inhibition in two of the three xenografts. When the radiation doses were adjusted to obtain a similar amount of radiation in the tumour c-17-1A was more effective in tumour growth inhibition in all three xenografts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R. D., Sharkey R. M., Haywood L., Natale A. M., Wong G. Y., Siegel J. A., Kennel S. J., Goldenberg D. M. Targeted therapy of athymic mice bearing GW-39 human colonic cancer micrometastases with 131I-labeled monoclonal antibodies. Cancer Res. 1992 Nov 1;52(21):6036–6044. [PubMed] [Google Scholar]
- Buchsbaum D. J., Brubaker P. G., Hanna D. E., Glatfelter A. A., Terry V. H., Guilbault D. M., Steplewski Z. Comparative binding and preclinical localization and therapy studies with radiolabeled human chimeric and murine 17-1A monoclonal antibodies. Cancer Res. 1990 Feb 1;50(3 Suppl):993s–999s. [PubMed] [Google Scholar]
- Chatal J. F., Saccavini J. C., Fumoleau P., Douillard J. Y., Curtet C., Kremer M., Le Mevel B., Koprowski H. Immunoscintigraphy of colon carcinoma. J Nucl Med. 1984 Mar;25(3):307–314. [PubMed] [Google Scholar]
- Chen T. R., Drabkowski D., Hay R. J., Macy M., Peterson W., Jr WiDr is a derivative of another colon adenocarcinoma cell line, HT-29. Cancer Genet Cytogenet. 1987 Jul;27(1):125–134. doi: 10.1016/0165-4608(87)90267-6. [DOI] [PubMed] [Google Scholar]
- Colcher D., Minelli M. F., Roselli M., Muraro R., Simpson-Milenic D., Schlom J. Radioimmunolocalization of human carcinoma xenografts with B72.3 second generation monoclonal antibodies. Cancer Res. 1988 Aug 15;48(16):4597–4603. [PubMed] [Google Scholar]
- Edwards D. P., Grzyb K. T., Dressler L. G., Mansel R. E., Zava D. T., Sledge G. W., Jr, McGuire W. L. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986 Mar;46(3):1306–1317. [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Haisma H. J., Hilgers J., Zurawski V. R., Jr Iodination of monoclonal antibodies for diagnosis and radiotherapy using a convenient one vial method. J Nucl Med. 1986 Dec;27(12):1890–1895. [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Hansen H. J., Goldenberg D. M., Newman E. S., Grebenau R., Sharkey R. M. Characterization of second-generation monoclonal antibodies against carcinoembryonic antigen. Cancer. 1993 Jun 1;71(11):3478–3485. doi: 10.1002/1097-0142(19930601)71:11<3478::aid-cncr2820711104>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Jurcic J. G., Scheinberg D. A. Recent developments in the radioimmunotherapy of cancer. Curr Opin Immunol. 1994 Oct;6(5):715–721. doi: 10.1016/0952-7915(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Juweid M., Neumann R., Paik C., Perez-Bacete M. J., Sato J., van Osdol W., Weinstein J. N. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992 Oct 1;52(19):5144–5153. [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Langmuir V. K., Mendonca H. L., Woo D. V. Comparisons between two monoclonal antibodies that bind to the same antigen but have differing affinities: uptake kinetics and 125I-antibody therapy efficacy in multicell spheroids. Cancer Res. 1992 Sep 1;52(17):4728–4734. [PubMed] [Google Scholar]
- Lindmo T., Boven E., Cuttitta F., Fedorko J., Bunn P. A., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984 Aug 3;72(1):77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- Linnenbach A. J., Seng B. A., Wu S., Robbins S., Scollon M., Pyrc J. J., Druck T., Huebner K. Retroposition in a family of carcinoma-associated antigen genes. Mol Cell Biol. 1993 Mar;13(3):1507–1515. doi: 10.1128/mcb.13.3.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellstedt H., Frödin J. E., Masucci G., Ragnhammar P., Fagerberg J., Hjelm A. L., Shetye J., Wersäll P., Osterborg A. The therapeutic use of monoclonal antibodies in colorectal carcinoma. Semin Oncol. 1991 Oct;18(5):462–477. [PubMed] [Google Scholar]
- Meredith R. F., LoBuglio A. F., Plott W. E., Orr R. A., Brezovich I. A., Russell C. D., Harvey E. B., Yester M. V., Wagner A. J., Spencer S. A. Pharmacokinetics, immune response, and biodistribution of iodine-131-labeled chimeric mouse/human IgG1,k 17-1A monoclonal antibody. J Nucl Med. 1991 Jun;32(6):1162–1168. [PubMed] [Google Scholar]
- Molthoff C. F., Calame J. J., Pinedo H. M., Boven E. Human ovarian cancer xenografts in nude mice: characterization and analysis of antigen expression. Int J Cancer. 1991 Jan 2;47(1):72–79. doi: 10.1002/ijc.2910470114. [DOI] [PubMed] [Google Scholar]
- Momburg F., Moldenhauer G., Hämmerling G. J., Möller P. Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissues. Cancer Res. 1987 Jun 1;47(11):2883–2891. [PubMed] [Google Scholar]
- Pak K. Y., Nedelman M. A., Fogler W. E., Tam S. H., Wilson E., Van Haarlem L. J., Colognola R., Warnaar S. O., Daddona P. E. Evaluation of the 323/A3 monoclonal antibody and the use of technetium-99m-labeled 323/A3 Fab' for the detection of pan adenocarcinoma. Int J Rad Appl Instrum B. 1991;18(5):483–497. doi: 10.1016/0883-2897(91)90109-x. [DOI] [PubMed] [Google Scholar]
- Quak J. J., Balm A. J., Brakkee J. G., Scheper R. J., Haisma H. J., Braakhuis B. J., Meijer C. J., Snow G. B. Localization and imaging of radiolabelled monoclonal antibody against squamous-cell carcinoma of the head and neck in tumor-bearing nude mice. Int J Cancer. 1989 Sep 15;44(3):534–538. doi: 10.1002/ijc.2910440327. [DOI] [PubMed] [Google Scholar]
- Riethmüller G., Schneider-Gädicke E., Schlimok G., Schmiegel W., Raab R., Höffken K., Gruber R., Pichlmaier H., Hirche H., Pichlmayr R. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes' C colorectal carcinoma. German Cancer Aid 17-1A Study Group. Lancet. 1994 May 14;343(8907):1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- Schlom J., Eggensperger D., Colcher D., Molinolo A., Houchens D., Miller L. S., Hinkle G., Siler K. Therapeutic advantage of high-affinity anticarcinoma radioimmunoconjugates. Cancer Res. 1992 Mar 1;52(5):1067–1072. [PubMed] [Google Scholar]
- Thampoe I. J., Ng J. S., Lloyd K. O. Biochemical analysis of a human epithelial surface antigen: differential cell expression and processing. Arch Biochem Biophys. 1988 Nov 15;267(1):342–352. doi: 10.1016/0003-9861(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Velders M. P., Litvinov S. V., Warnaar S. O., Gorter A., Fleuren G. J., Zurawski V. R., Jr, Coney L. R. New chimeric anti-pancarcinoma monoclonal antibody with superior cytotoxicity-mediating potency. Cancer Res. 1994 Apr 1;54(7):1753–1759. [PubMed] [Google Scholar]
- Wahl R. L. Experimental radioimmunotherapy. A brief overview. Cancer. 1994 Feb 1;73(3 Suppl):989–992. doi: 10.1002/1097-0142(19940201)73:3+<989::aid-cncr2820731336>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]