Abstract
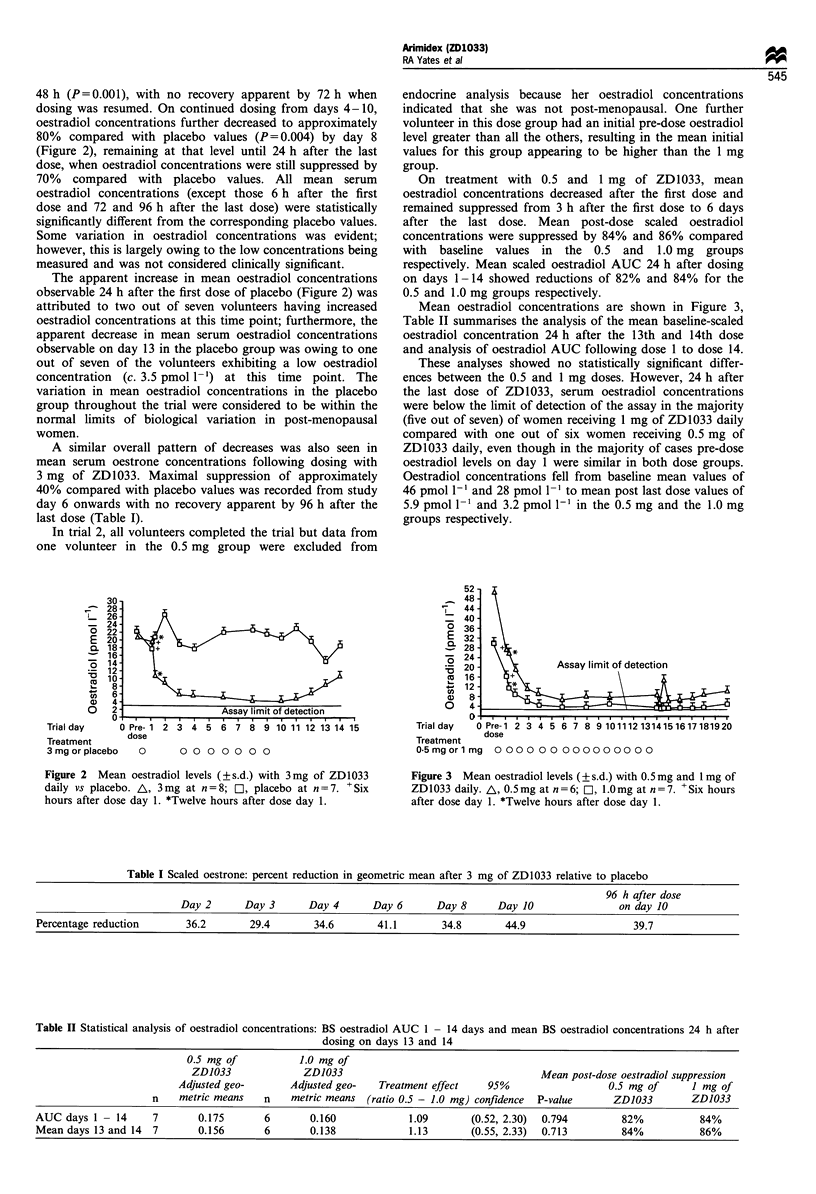
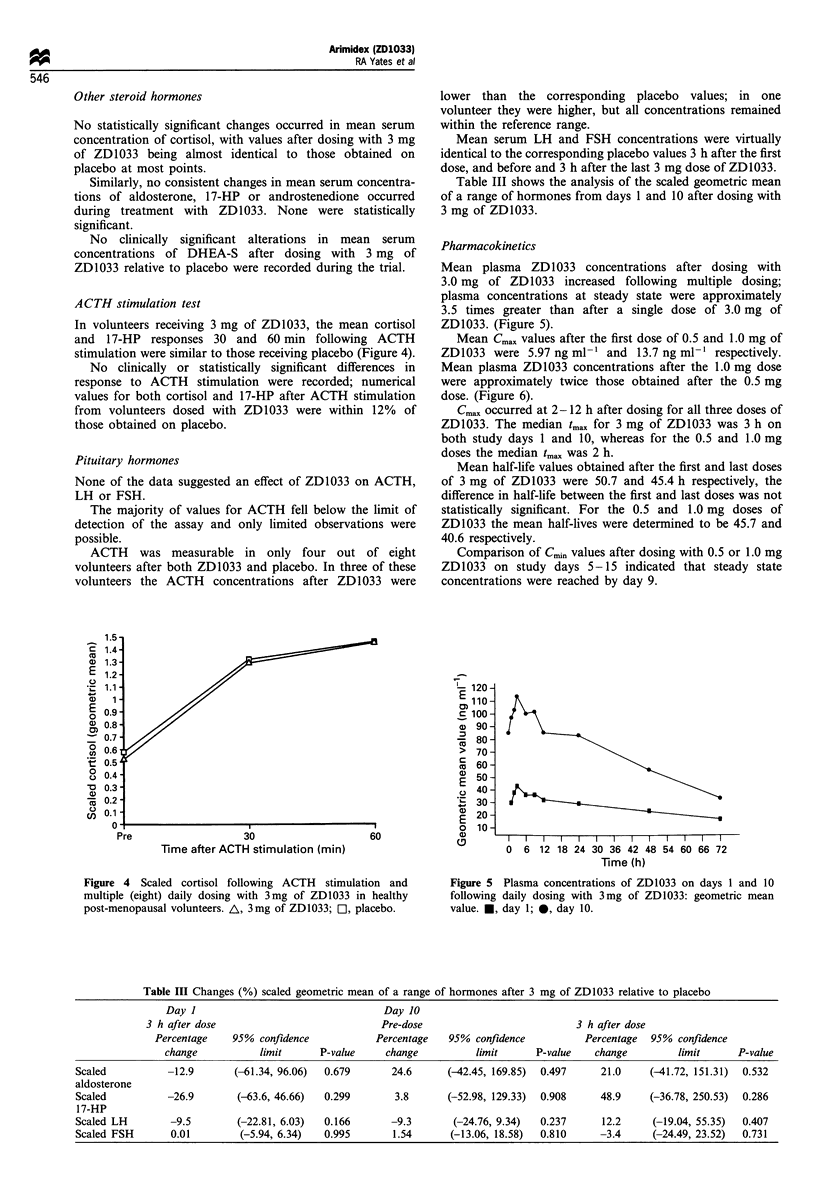
Two multiple-dose studies were conducted in healthy post-menopausal female volunteers to investigate the pharmacokinetics and effects on endocrinology of Arimidex (ZD1033). Volunteers in the first trial were dosed with 3 mg of ZD1033 daily over 10 days to assess the effects on endocrinology of ZD1033 and establish a pharmacokinetic profile. In the second trial volunteers received 14 daily doses of either 0.5 or 1.0 mg of ZD1033 to assess the pharmacokinetics of ZD1033 and the effects of low doses of ZD1033 on serum oestradiol concentrations. Following multiple dosing a significant reduction in the concentration of serum oestradiol of approximately 80% of baseline was obtained with all three doses; no recovery in oestradiol was apparent for up to 144 h after the last dose. There was no overall difference in the level of oestradiol suppression between the 0.5 or 1.0 mg doses of ZD1033. However, comparison of the number of volunteers with oestradiol concentrations below the limits of detection of the assay, 24 h after the last dose of ZD1033, suggested that 1.0 mg was the minimal dose required for maximal suppression of oestradiol. No significant effect was recorded on serum concentrations of gonadotrophins over the dosing period. Serum concentrations of a range of adrenal steroids were not affected by administration of ZD1033; furthermore, steroid response to standard adrenocorticotrophic hormone (ACTH) challenge was unimpaired by ZD1033. Together these data demonstrate the potency, tolerability and selectivity of ZD1033. The pharmacokinetic profile of ZD1033 supports its use as a once-daily treatment given orally.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demers L. M., Lipton A., Harvey H. A., Hanagan J., Mulagha M., Santen R. J. The effects of long term fadrozole hydrochloride treatment in patients with advanced stage breast cancer. J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):683–685. doi: 10.1016/0960-0760(93)90282-2. [DOI] [PubMed] [Google Scholar]
- Dowsett M., Goss P. E., Powles T. J., Hutchinson G., Brodie A. M., Jeffcoate S. L., Coombes R. C. Use of the aromatase inhibitor 4-hydroxyandrostenedione in postmenopausal breast cancer: optimization of therapeutic dose and route. Cancer Res. 1987 Apr 1;47(7):1957–1961. [PubMed] [Google Scholar]
- Goss P. E., Gwyn K. M. Current perspectives on aromatase inhibitors in breast cancer. J Clin Oncol. 1994 Nov;12(11):2460–2470. doi: 10.1200/JCO.1994.12.11.2460. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Dowsett M., Jeffcoate S. L., Smith I. E. Aminoglutethimide dose and hormone suppression in advanced breast cancer. Eur J Cancer Clin Oncol. 1983 Apr;19(4):493–498. doi: 10.1016/0277-5379(83)90112-8. [DOI] [PubMed] [Google Scholar]
- Höffken K. Experience with aromatase inhibitors in the treatment of advanced breast cancer. Cancer Treat Rev. 1993 Apr;19 (Suppl B):37–44. doi: 10.1016/0305-7372(93)90006-d. [DOI] [PubMed] [Google Scholar]
- Höffken K., Jonat W., Possinger K., Kölbel M., Kunz T., Wagner H., Becher R., Callies R., Friederich P., Willmanns W. Aromatase inhibition with 4-hydroxyandrostenedione in the treatment of postmenopausal patients with advanced breast cancer: a phase II study. J Clin Oncol. 1990 May;8(5):875–880. doi: 10.1200/JCO.1990.8.5.875. [DOI] [PubMed] [Google Scholar]
- Iveson T. J., Smith I. E., Ahern J., Smithers D. A., Trunet P. F., Dowsett M. Phase I study of the oral nonsteroidal aromatase inhibitor CGS 20267 in postmenopausal patients with advanced breast cancer. Cancer Res. 1993 Jan 15;53(2):266–270. [PubMed] [Google Scholar]
- Johnston S. R., Smith I. E., Doody D., Jacobs S., Robertshaw H., Dowsett M. Clinical and endocrine effects of the oral aromatase inhibitor vorozole in postmenopausal patients with advanced breast cancer. Cancer Res. 1994 Nov 15;54(22):5875–5881. [PubMed] [Google Scholar]
- Miller W. R. Aromatase inhibitors in the treatment of advanced breast cancer. Cancer Treat Rev. 1989 Jun;16(2):83–93. doi: 10.1016/0305-7372(89)90012-1. [DOI] [PubMed] [Google Scholar]
- Muss H. B. Endocrine therapy for advanced breast cancer: a review. Breast Cancer Res Treat. 1992;21(1):15–26. doi: 10.1007/BF01811960. [DOI] [PubMed] [Google Scholar]
- Plourde P. V., Dyroff M., Dukes M. Arimidex: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30(1):103–111. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- Santen R. J. Clinical use of aromatase inhibitors in human breast carcinoma. J Steroid Biochem Mol Biol. 1991;40(1-3):247–253. doi: 10.1016/0960-0760(91)90189-c. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Demers L. M., Lynch J., Harvey H., Lipton A., Mulagha M., Hanagan J., Garber J. E., Henderson I. C., Navari R. M. Specificity of low dose fadrozole hydrochloride (CGS 16949A) as an aromatase inhibitor. J Clin Endocrinol Metab. 1991 Jul;73(1):99–106. doi: 10.1210/jcem-73-1-99. [DOI] [PubMed] [Google Scholar]
- Stein R. C., Dowsett M., Hedley A., Davenport J., Gazet J. C., Ford H. T., Coombes R. C. Treatment of advanced breast cancer in postmenopausal women with 4-hydroxyandrostenedione. Cancer Chemother Pharmacol. 1990;26(1):75–78. doi: 10.1007/BF02940300. [DOI] [PubMed] [Google Scholar]



