Abstract
The effects of photodynamic treatment (PDT) on venules include vascular leakage accompanied by oedema formation, vasoconstriction and blood flow stasis. The goal of this study was to gain insight into the mechanism underlying these vascular events by studying one of the earliest observations after PDT, granulocyte adhesion, in an in vitro model. For this purpose human umbilical vein endothelial cells (HUVECs) preincubated with Photofrin II (PII) were illuminated with red light and incubated with neutrophils. PDT led to a dramatic change in the morphology of the endothelial cells. Clearly, neutrophils adhered to the subendothelial matrix and their adherence coincided with an increase in the percentage of exposed subendothelial matrix by the gradual contraction of endothelial cells. Furthermore, the increase in adherence was dependent on drug dose, illumination time and the time delay after PDT. The neutrophil adherence could be inhibited by anti-beta2-integrin antibodies, which suggests that the alphaL-, alphaM- or alphaX-beta2 receptors of the neutrophil mediated this phenomenon. At 4 degrees C or by preincubation of the neutrophils with staurosporin, their adherence to the subendothelial matrix exposed by PDT of endothelial cells could be prevented. Apparently, activation of the beta2-integrin receptor by interaction with the subendothelial matrix is necessary for the increased binding of neutrophils. Taken together, these in vitro findings suggest that the PDT-induced contraction of the endothelial cells permits neutrophil adherence to the subendothelial matrix. It is conceivable that a similar mechanism contributes to the initial adherence of granulocytes to the vessel wall as observed after PDT in vivo.
Full text
PDF

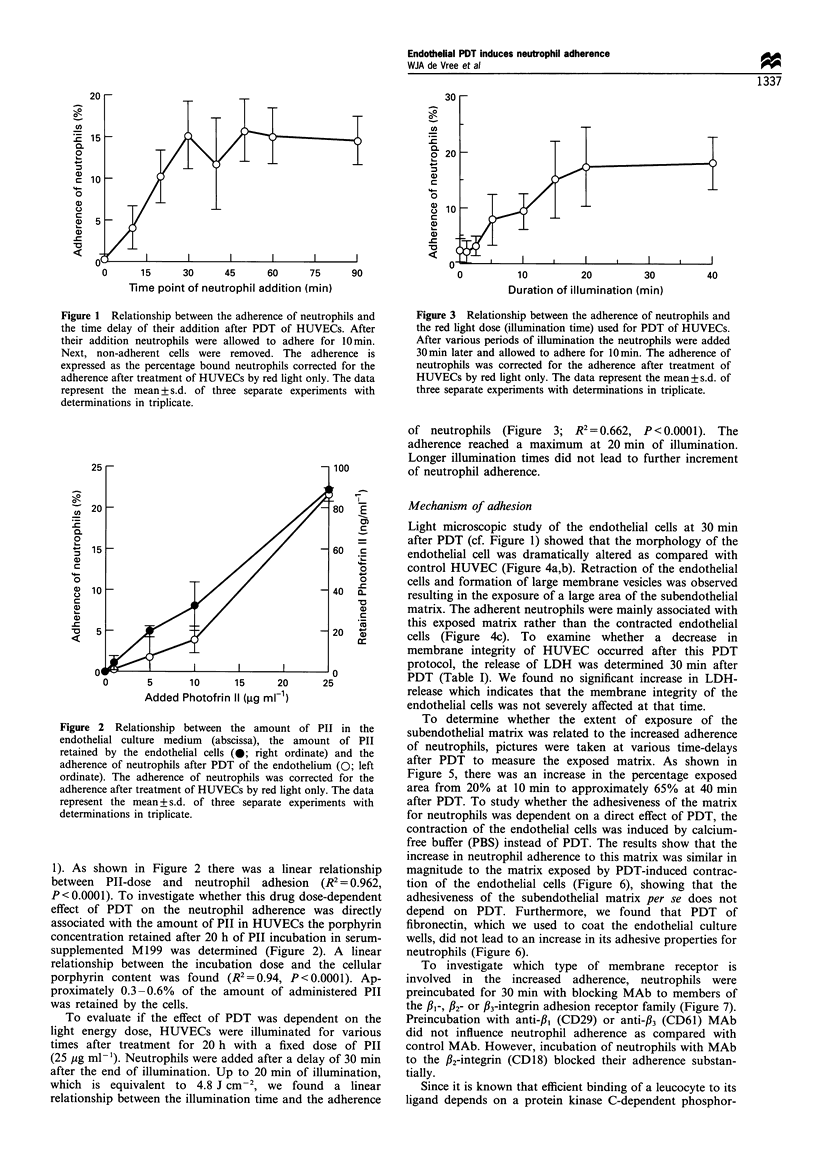
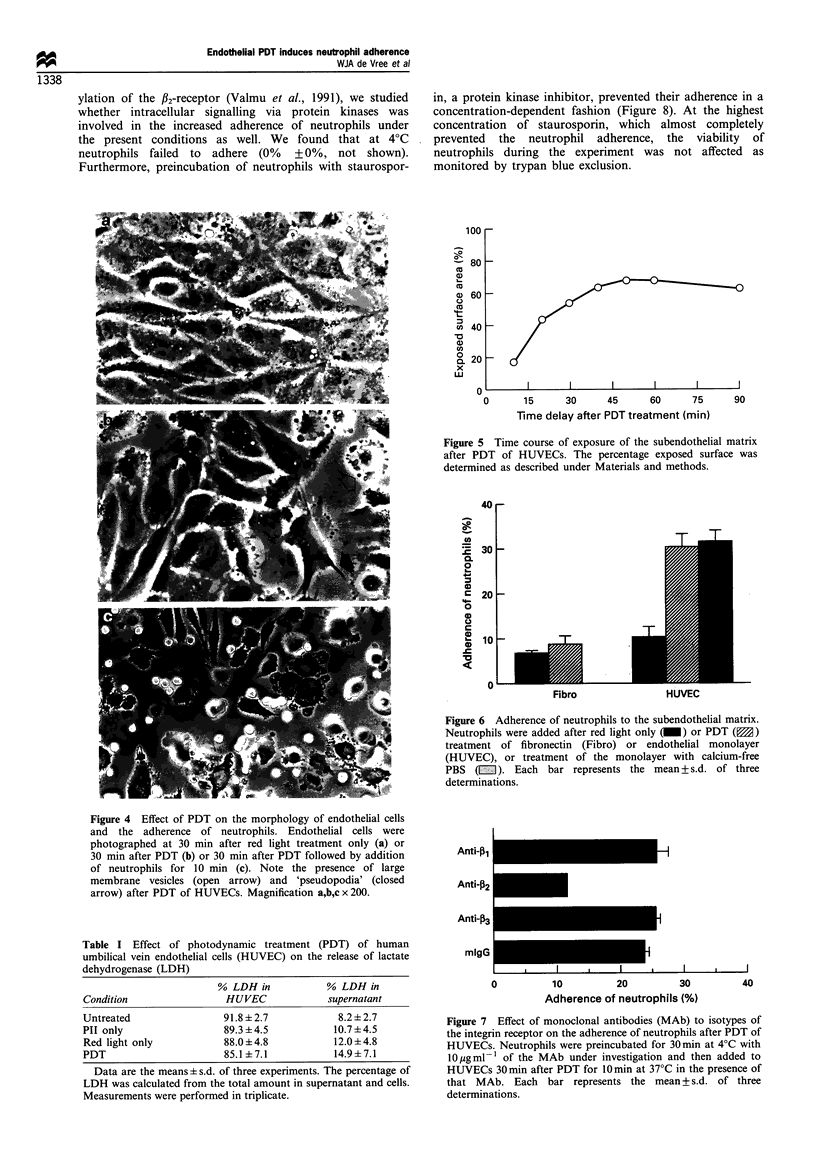
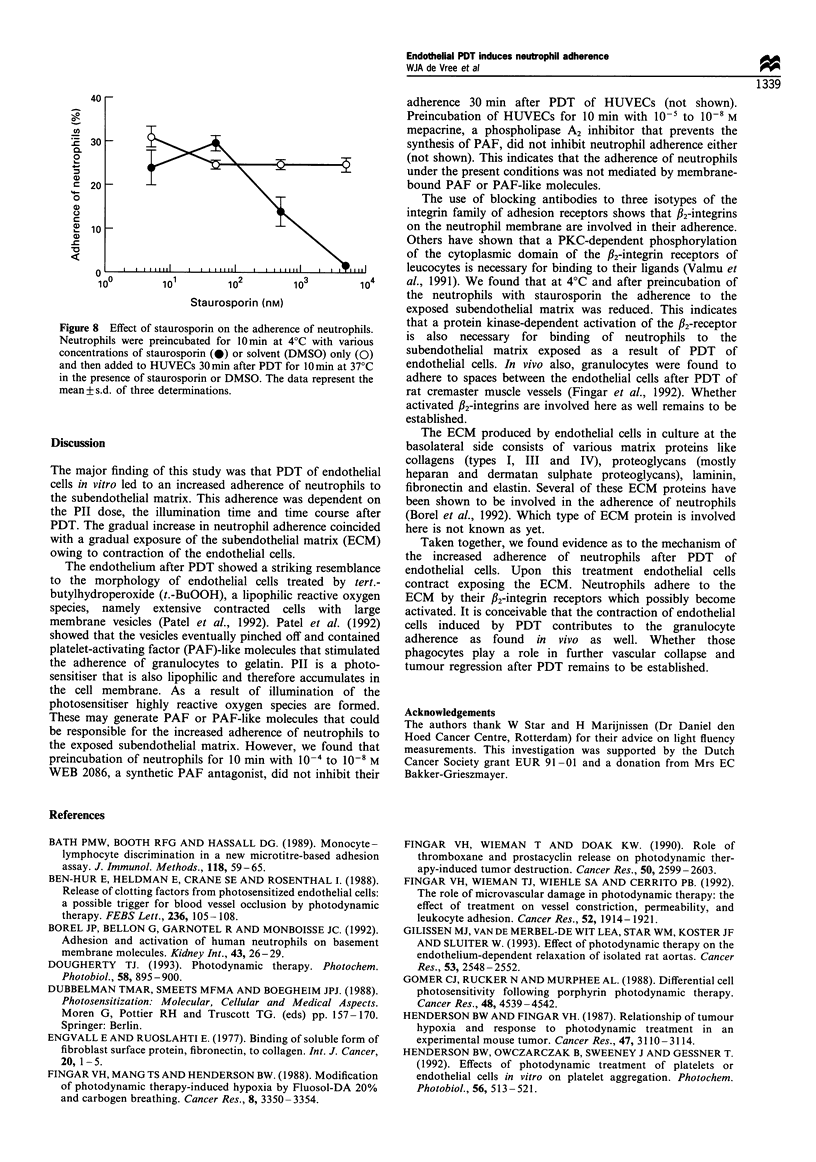

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bath P. M., Booth R. F., Hassall D. G. Monocyte-lymphocyte discrimination in a new microtitre-based adhesion assay. J Immunol Methods. 1989 Mar 10;118(1):59–65. doi: 10.1016/0022-1759(89)90053-7. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Heldman E., Crane S. W., Rosenthal I. Release of clotting factors from photosensitized endothelial cells: a possible trigger for blood vessel occlusion by photodynamic therapy. FEBS Lett. 1988 Aug 15;236(1):105–108. doi: 10.1016/0014-5793(88)80294-1. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photodynamic therapy. Photochem Photobiol. 1993 Dec;58(6):895–900. doi: 10.1111/j.1751-1097.1993.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Fingar V. H., Mang T. S., Henderson B. W. Modification of photodynamic therapy-induced hypoxia by fluosol-DA (20%) and carbogen breathing in mice. Cancer Res. 1988 Jun 15;48(12):3350–3354. [PubMed] [Google Scholar]
- Fingar V. H., Wieman T. J., Doak K. W. Role of thromboxane and prostacyclin release on photodynamic therapy-induced tumor destruction. Cancer Res. 1990 May 1;50(9):2599–2603. [PubMed] [Google Scholar]
- Gilissen M. J., van de Merbel-de Wit L. E., Star W. M., Koster J. F., Sluiter W. Effect of photodynamic therapy on the endothelium-dependent relaxation of isolated rat aortas. Cancer Res. 1993 Jun 1;53(11):2548–2552. [PubMed] [Google Scholar]
- Gomer C. J., Rucker N., Murphree A. L. Differential cell photosensitivity following porphyrin photodynamic therapy. Cancer Res. 1988 Aug 15;48(16):4539–4542. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J., Pettersen E. O., Christensen T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br J Cancer. 1979 Apr;39(4):398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McIntyre T. M. Novel leukocyte agonists are released by endothelial cells exposed to peroxide. J Biol Chem. 1992 Jul 25;267(21):15168–15175. [PubMed] [Google Scholar]
- Spikes J. D. Porphyrins and related compounds as photodynamic sensitizers. Ann N Y Acad Sci. 1975 Apr 15;244:496–508. doi: 10.1111/j.1749-6632.1975.tb41550.x. [DOI] [PubMed] [Google Scholar]
- Star W. M., Marijnissen H. P., van den Berg-Blok A. E., Versteeg J. A., Franken K. A., Reinhold H. S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986 May;46(5):2532–2540. [PubMed] [Google Scholar]
- Valmu L., Autero M., Siljander P., Patarroyo M., Gahmberg C. G. Phosphorylation of the beta-subunit of CD11/CD18 integrins by protein kinase C correlates with leukocyte adhesion. Eur J Immunol. 1991 Nov;21(11):2857–2862. doi: 10.1002/eji.1830211130. [DOI] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]