Abstract
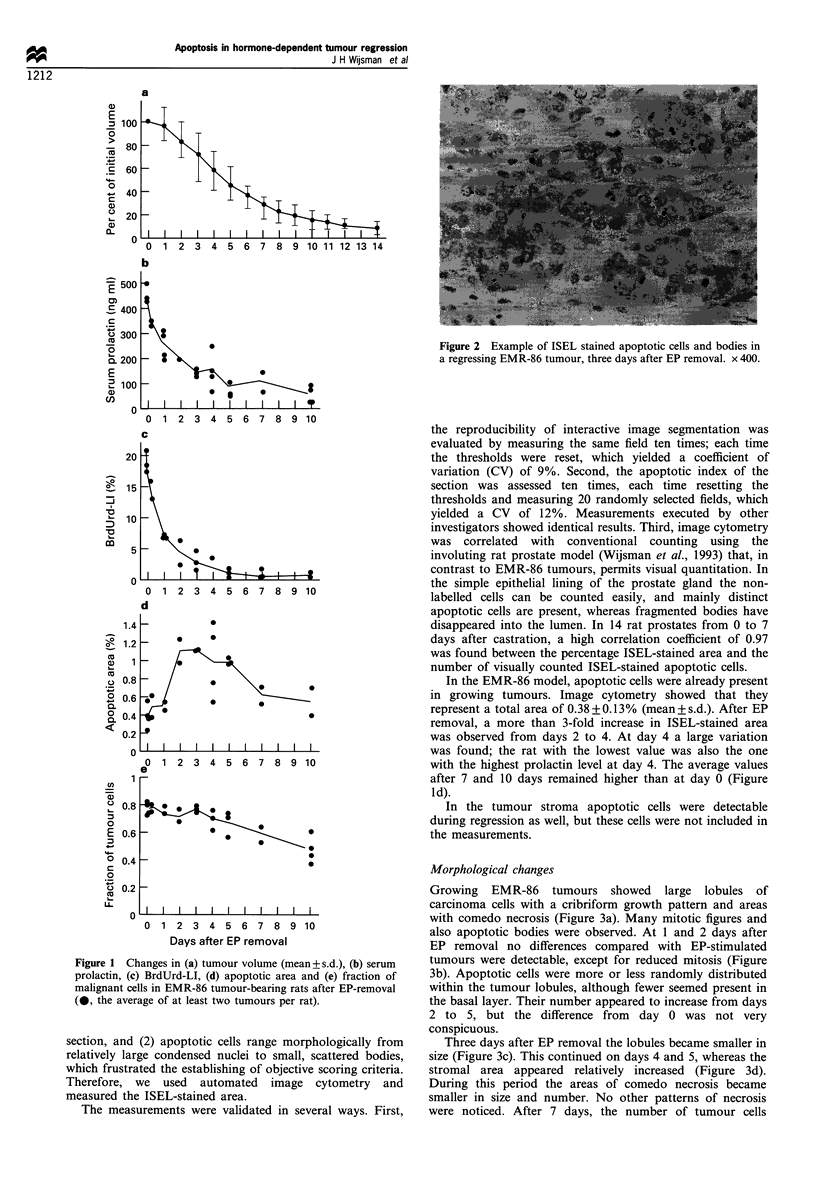
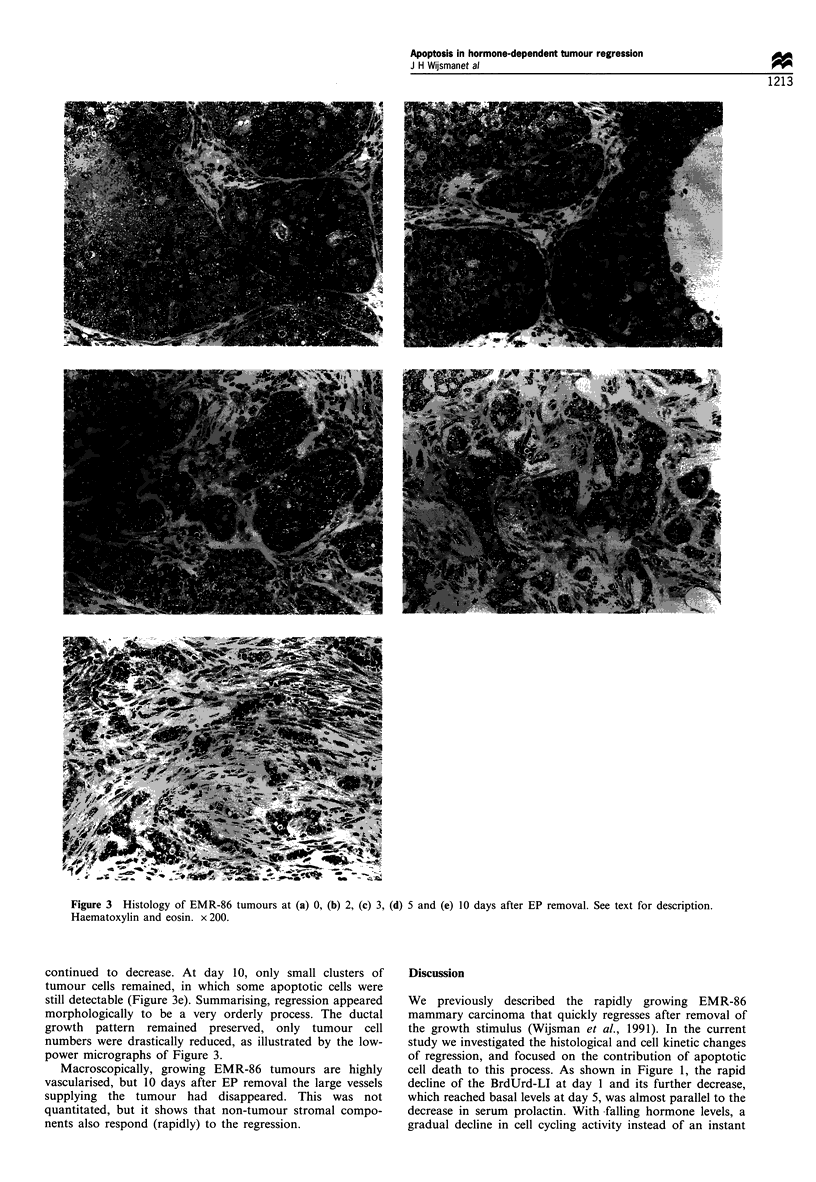
Growth of the transplantable EMR-86 rat mammary carcinoma depends on elevated prolactin levels which are induced by oestrogenic stimulation of the pituitary. We investigated histological and cell kinetic changes during tumour regression after removal of implanted oestrogen pellets (EP), and we especially focused on the role of apoptosis. After EP removal, serum prolactin decreased to basal levels in 5 days, reaching its largest depletion during the first day. Similarly, S-phase cell fractions, assessed by bromodeoxyuridine (BrdUrd) incorporation, decreased to half the initial value during the first day and developed into a gradual decrease to basal levels thereafter. Within 10 days, tumour volumes were reduced to 20% without striking changes in tissue architecture. To quantify apoptosis, we applied a method that stains DNA breaks in tissue sections and subsequently measured the stained area by automated image cytometry. This procedure was necessary, as the subtle changes could not be detected by histological examination alone. One day after the rapid decline of the S-phase fraction, a 3-fold increase in apoptotic area was observed that remained for about 3 days and then gradually decreased. This correlated with the histologically observed reduction of tumour cells. In spite of the major cell loss, regression came to a halt after about 10 days. The surviving cell fraction is discussed within the context of a stem cell hypothesis, in which tumour cells with stem cell characteristics are less susceptible to hormone-induced apoptosis than their (non-stem) daughter cells. This notion has implications for the eradication of residual tumour cells, because a diminished susceptibility might also apply to apoptosis induced by radio- or chemotherapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Colombel M., Olsson C. A., Ng P. Y., Buttyan R. Hormone-regulated apoptosis results from reentry of differentiated prostate cells onto a defective cell cycle. Cancer Res. 1992 Aug 15;52(16):4313–4319. [PubMed] [Google Scholar]
- Cutts J. H., Froude G. C. Regression of estrone-induced mammary tumors in the rat. Cancer Res. 1968 Dec;28(12):2413–2418. [PubMed] [Google Scholar]
- Dethlefsen L. A., Prewitt J. M., Mendelsohn M. L. Analysis of tumor growth curves. J Natl Cancer Inst. 1968 Feb;40(2):389–405. doi: 10.1093/jnci/40.2.389. [DOI] [PubMed] [Google Scholar]
- English H. F., Kyprianou N., Isaacs J. T. Relationship between DNA fragmentation and apoptosis in the programmed cell death in the rat prostate following castration. Prostate. 1989;15(3):233–250. doi: 10.1002/pros.2990150304. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Anderson T. J. Ultrastructural observations on cell death by apoptosis in the "resting" human breast. Virchows Arch A Pathol Anat Histol. 1981;393(2):193–203. doi: 10.1007/BF00431076. [DOI] [PubMed] [Google Scholar]
- Guenette R. S., Corbeil H. B., Léger J., Wong K., Mézl V., Mooibroek M., Tenniswood M. Induction of gene expression during involution of the lactating mammary gland of the rat. J Mol Endocrinol. 1994 Feb;12(1):47–60. doi: 10.1677/jme.0.0120047. [DOI] [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Losonczy I., Berghoffer B. Mammary tumor regression. I. Physiopathologic characteristics of hormone-dependent tissue. J Natl Cancer Inst. 1972 Nov;49(5):1333–1348. [PubMed] [Google Scholar]
- Gullino P. M., Lanzerotti R. H. Mammary tumor regression. II. Autophagy of neoplastic cells. J Natl Cancer Inst. 1972 Nov;49(5):1349–1356. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Lancaster S., English H. F., Demers L. M., Manni A. Kinetic and morphometric responses of heterogeneous populations of experimental breast cancer cells in vivo. Cancer Res. 1988 Jun 1;48(11):3276–3281. [PubMed] [Google Scholar]
- Landström M., Damber J. E., Bergh A. Prostatic tumor regrowth after initially successful castration therapy may be related to a decreased apoptotic cell death rate. Cancer Res. 1994 Aug 15;54(16):4281–4284. [PubMed] [Google Scholar]
- Rennie P. S., Bruchovsky N., Buttyan R., Benson M., Cheng H. Gene expression during the early phases of regression of the androgen-dependent Shionogi mouse mammary carcinoma. Cancer Res. 1988 Nov 15;48(22):6309–6312. [PubMed] [Google Scholar]
- Sandford N. L., Searle J. W., Kerr J. F. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984 Oct;16(4):406–410. doi: 10.3109/00313028409084731. [DOI] [PubMed] [Google Scholar]
- Strange R., Li F., Saurer S., Burkhardt A., Friis R. R. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992 May;115(1):49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Bennett R. E., Kerr J. F. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989 May;185(1):19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Wijsman J. H., Cornelisse C. J., Keijzer R., van de Velde C. J., van Dierendonck J. H. A prolactin-dependent, metastasising rat mammary carcinoma as a model for endocrine-related tumour dormancy. Br J Cancer. 1991 Sep;64(3):463–468. doi: 10.1038/bjc.1991.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Wijsman J. H., Van Dierendonck J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Immunoreactivity of proliferating cell nuclear antigen compared with bromodeoxyuridine incorporation in normal and neoplastic rat tissue. J Pathol. 1992 Sep;168(1):75–83. doi: 10.1002/path.1711680113. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis and the regulation of cell numbers in normal and neoplastic tissues: an overview. Cancer Metastasis Rev. 1992 Sep;11(2):95–103. doi: 10.1007/BF00048057. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]




