Abstract
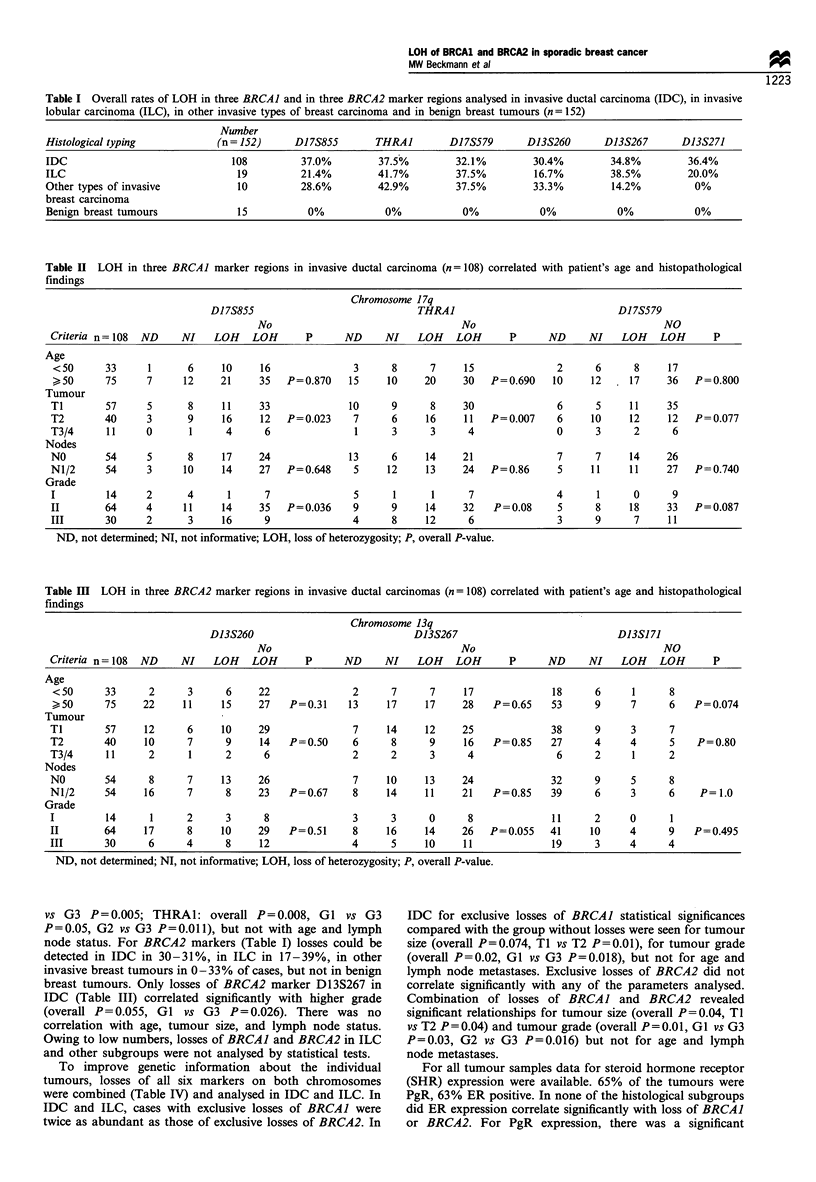
The development of familial and sporadic breast cancer is based on genetic alterations of tumour-suppressor genes, for which loss of heterozygosity (LOH) is one mechanism of gene inactivation. To investigate LOH of BRCA1 (17q21) and BRCA2 (13-q12-13) in sporadic breast cancer, polymerase chain reaction (PCR)-based fluorescent DNA technology for detection of microsatellite polymorphisms was applied. A total of 137 breast cancer and 15 benign breast specimens with matched normal tissue were examined. Fluorescent-labelled PCR products were analysed in an automated DNA sequencer (ALFTM Pharmacia). Losses at both loci were correlated with different histological types, age, tumour size, lymph node status, grading and steroid hormone receptor expression, [SHR: oestrogen receptor (ER), progesterone receptor (PgR)]. For BRCA1 (D17S855, THRA1, D17S579) losses could be detected in invasive ductal carcinoma (IDC; n = 108) in 32-38%, invasive lobular carcinoma (ILC; n = 19) in 21-42% depending on the marker applied, but not in benign breast tumours (n = 15). Losses of BRCA1 markers correlated with larger tumour size, higher grade, and PgR expression. For BRCA2 (D13S260, D13S267, D13S171) losses could be detected in 108 IDCs in 30-38%, in 19 ILCs in 17-39% depending on the marker applied, but not in benign breast tumours. Losses of BRCA2 markers correlated only with higher grade. Microsatellite analyses combined with detection of fluorescent-labelled PCR products by an automated laser DNA sequencer can be used for routine determination of LOH. In sporadic breast cancer, LOH of BRCA1 of BRCA2 does not add decisive prognostic value as stated for familial breast cancer.
Full text
PDF

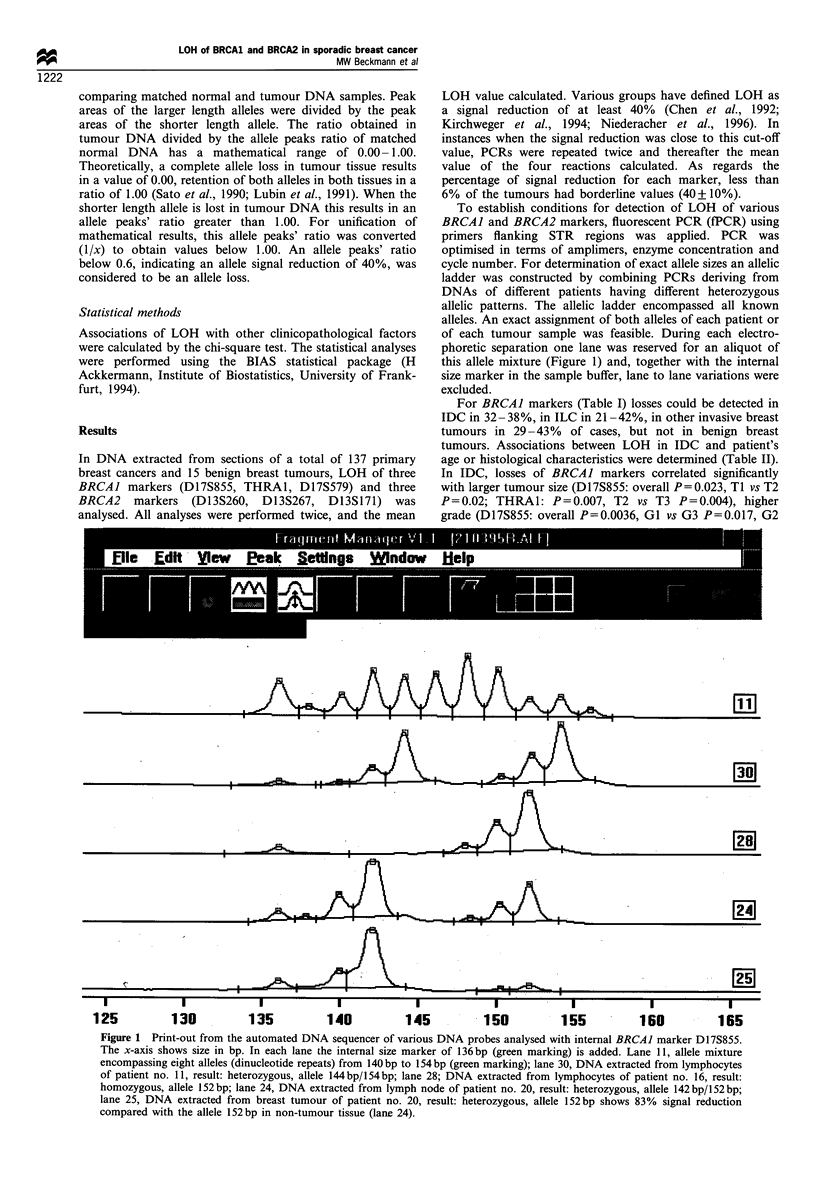



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen T. I., Gaustad A., Ottestad L., Farrants G. W., Nesland J. M., Tveit K. M., Børresen A. L. Genetic alterations of the tumour suppressor gene regions 3p, 11p, 13q, 17p, and 17q in human breast carcinomas. Genes Chromosomes Cancer. 1992 Mar;4(2):113–121. doi: 10.1002/gcc.2870040203. [DOI] [PubMed] [Google Scholar]
- Black D. M. The genetics of breast cancer. Eur J Cancer. 1994;30A(13):1957–1961. doi: 10.1016/0959-8049(94)00386-j. [DOI] [PubMed] [Google Scholar]
- Boyd J. BRCA1: more than a hereditary breast cancer gene? Nat Genet. 1995 Apr;9(4):335–336. doi: 10.1038/ng0495-335. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Kurisu W., Ljung B. M., Goldman E. S., Moore D., 2nd, Smith H. S. Heterogeneity for allelic loss in human breast cancer. J Natl Cancer Inst. 1992 Apr 1;84(7):506–510. doi: 10.1093/jnci/84.7.506. [DOI] [PubMed] [Google Scholar]
- Collins N., McManus R., Wooster R., Mangion J., Seal S., Lakhani S. R., Ormiston W., Daly P. A., Ford D., Easton D. F. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12-13. Oncogene. 1995 Apr 20;10(8):1673–1675. [PubMed] [Google Scholar]
- Cropp C. S., Champeme M. H., Lidereau R., Callahan R. Identification of three regions on chromosome 17q in primary human breast carcinomas which are frequently deleted. Cancer Res. 1993 Dec 1;53(23):5617–5619. [PubMed] [Google Scholar]
- Deng G., Chen L. C., Schott D. R., Thor A., Bhargava V., Ljung B. M., Chew K., Smith H. S. Loss of heterozygosity and p53 gene mutations in breast cancer. Cancer Res. 1994 Jan 15;54(2):499–505. [PubMed] [Google Scholar]
- Devilee P., Cornelisse C. J. Somatic genetic changes in human breast cancer. Biochim Biophys Acta. 1994 Dec 30;1198(2-3):113–130. doi: 10.1016/0304-419x(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Ford D., Easton D. F., Bishop D. T., Narod S. A., Goldgar D. E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Söderkvist P., Marks J. R., Iglehart J. D., Cochran C., Barrett J. C., Wiseman R. W. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992 May 1;52(9):2624–2627. [PubMed] [Google Scholar]
- Hall J. M., Friedman L., Guenther C., Lee M. K., Weber J. L., Black D. M., King M. C. Closing in on a breast cancer gene on chromosome 17q. Am J Hum Genet. 1992 Jun;50(6):1235–1242. [PMC free article] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Hogervorst F. B., Cornelis R. S., Bout M., van Vliet M., Oosterwijk J. C., Olmer R., Bakker B., Klijn J. G., Vasen H. F., Meijers-Heijboer H. Rapid detection of BRCA1 mutations by the protein truncation test. Nat Genet. 1995 Jun;10(2):208–212. doi: 10.1038/ng0695-208. [DOI] [PubMed] [Google Scholar]
- Hosking L., Trowsdale J., Nicolai H., Solomon E., Foulkes W., Stamp G., Signer E., Jeffreys A. A somatic BRCA1 mutation in an ovarian tumour. Nat Genet. 1995 Apr;9(4):343–344. doi: 10.1038/ng0495-343. [DOI] [PubMed] [Google Scholar]
- Jacquemier J., Eisinger F., Birnbaum D., Sobol H. Histoprognostic grade in BRCA1-associated breast cancer. Lancet. 1995 Jun 10;345(8963):1503–1503. doi: 10.1016/s0140-6736(95)91060-3. [DOI] [PubMed] [Google Scholar]
- Kirchweger R., Zeillinger R., Schneeberger C., Speiser P., Louason G., Theillet C. Patterns of allele losses suggest the existence of five distinct regions of LOH on chromosome 17 in breast cancer. Int J Cancer. 1994 Jan 15;56(2):193–199. doi: 10.1002/ijc.2910560208. [DOI] [PubMed] [Google Scholar]
- Knyazev P. G., Imyanitov E. N., Chernitsa O. I., Nikiforova I. F. Loss of heterozygosity at chromosome 17p is associated with HER-2 amplification and lack of nodal involvement in breast cancer. Int J Cancer. 1993 Jan 2;53(1):11–16. doi: 10.1002/ijc.2910530104. [DOI] [PubMed] [Google Scholar]
- Lalle P., De Latour M., Rio P., Bignon Y. J. Detection of allelic losses on 17q12-q21 chromosomal region in benign lesions and malignant tumors occurring in a familial context. Oncogene. 1994 Feb;9(2):437–442. [PubMed] [Google Scholar]
- Liu E. T., He M., Rajgopal U. Differential polymerase chain reaction in the analysis of gene dosage. Semin Cancer Biol. 1993 Feb;4(1):47–58. [PubMed] [Google Scholar]
- Lubin M. B., Elashoff J. D., Wang S. J., Rotter J. I., Toyoda H. Precise gene dosage determination by polymerase chain reaction: theory, methodology, and statistical approach. Mol Cell Probes. 1991 Aug;5(4):307–317. doi: 10.1016/0890-8508(91)90054-n. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Lynch J., Conway T., Watson P., Feunteun J., Lenoir G., Narod S., Fitzgibbons R., Jr Hereditary breast cancer and family cancer syndromes. World J Surg. 1994 Jan-Feb;18(1):21–31. doi: 10.1007/BF00348188. [DOI] [PubMed] [Google Scholar]
- Merajver S. D., Pham T. M., Caduff R. F., Chen M., Poy E. L., Cooney K. A., Weber B. L., Collins F. S., Johnston C., Frank T. S. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995 Apr;9(4):439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- Sato T., Tanigami A., Yamakawa K., Akiyama F., Kasumi F., Sakamoto G., Nakamura Y. Allelotype of breast cancer: cumulative allele losses promote tumor progression in primary breast cancer. Cancer Res. 1990 Nov 15;50(22):7184–7189. [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Shibata D. Extraction of DNA from paraffin-embedded tissue for analysis by polymerase chain reaction: new tricks from an old friend. Hum Pathol. 1994 Jun;25(6):561–563. doi: 10.1016/0046-8177(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Thompson M. E., Jensen R. A., Obermiller P. S., Page D. L., Holt J. T. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995 Apr;9(4):444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- el-Ashry D., Lippman M. E. Molecular biology of breast carcinoma. World J Surg. 1994 Jan-Feb;18(1):12–20. doi: 10.1007/BF00348187. [DOI] [PubMed] [Google Scholar]