Abstract
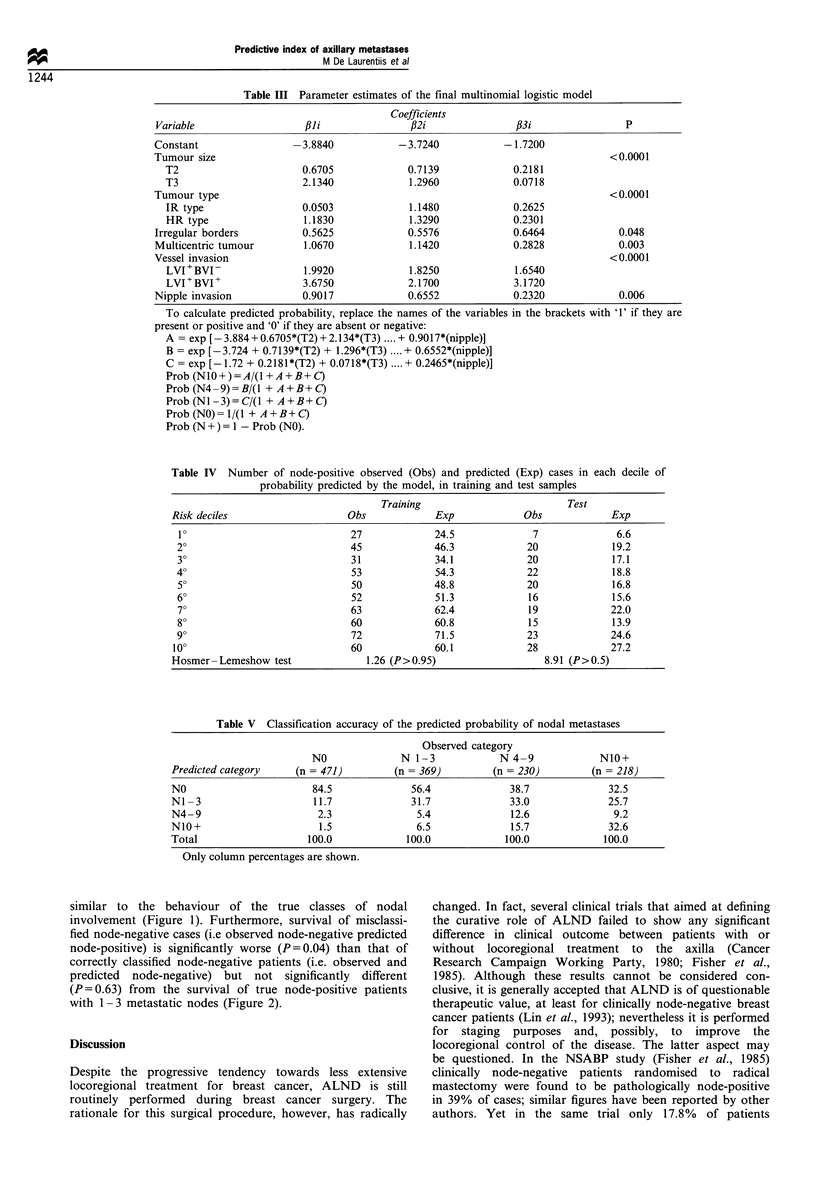
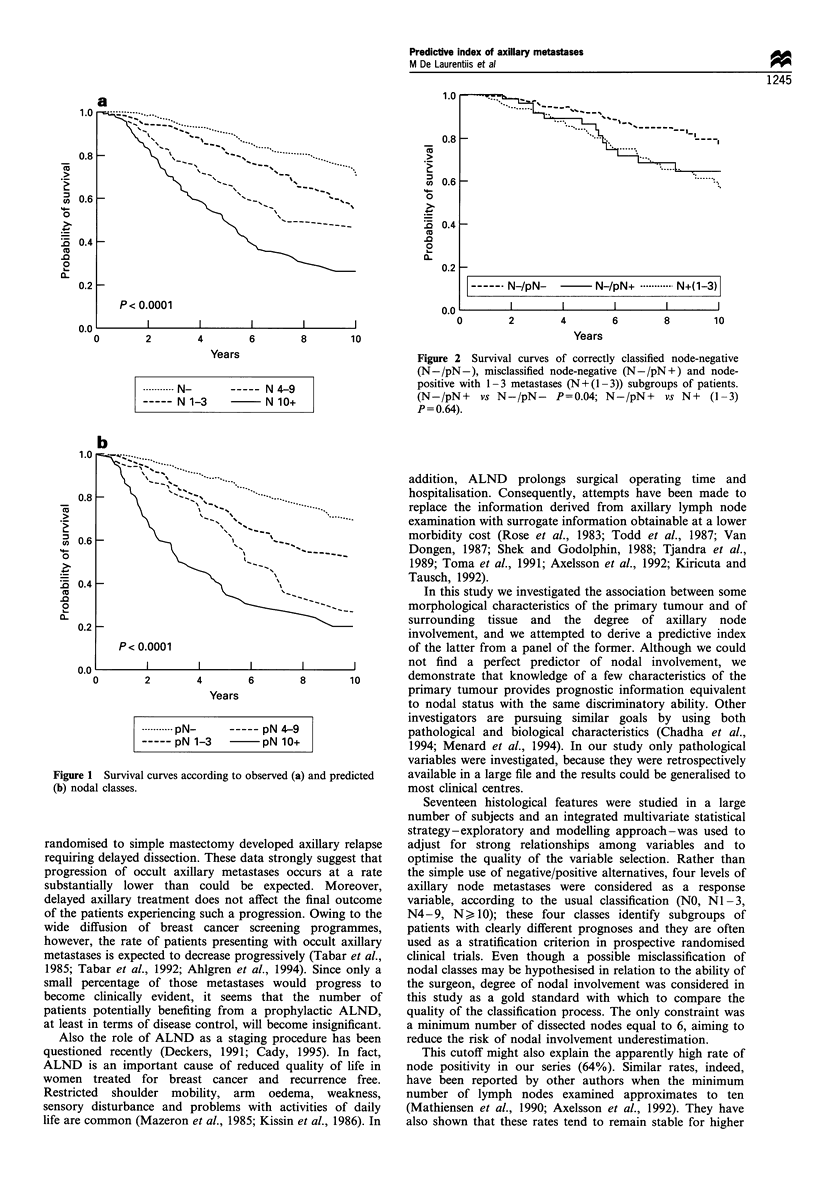
We investigated the association between pathological characteristics of primary breast cancer and degree of axillary nodal involvement and obtained a predictive index of the latter from the former. In 2076 cases, 17 histological features, including primary tumour and local invasion variables, were recorded. The whole sample was randomly split in a training (75% of cases) and a test sample. Simple and multiple correspondence analysis were used to select the variables to enter in a multinomial logit model to build an index predictive of the degree of nodal involvement. The response variable was axillary nodal status coded in four classes (N0, N1-3, N4-9, N > or = 10). The predictive index was then evaluated by testing goodness-of-fit and classification accuracy. Covariates significantly associated with nodal status were tumour size (P < 0.0001), tumour type (P < 0.0001), type of border (P = 0.048), multicentricity (P = 0.003), invasion of lymphatic and blood vessels (P < 0.0001) and nipple invasion (P = 0.006). Goodness-of-fit was validated by high concordance between observed and expected number of cases in each decile of predicted probability in both training and test samples. Classification accuracy analysis showed that true node-positive cases were well recognised (84.5%), but there was no clear distinction among the classes of node-positive cases. However, 10 year survival analysis showed a superimposible prognostic behaviour between predicted and observed nodal classes. Moreover, misclassified node-negative patients (i.e. those who are predicted positive) showed an outcome closer to patients with 1-3 metastatic nodes than to node-negative ones. In conclusion, the index cannot completely substitute for axillary node information, but it is a predictor of prognosis as accurate as nodal involvement and identifies a subgroup of node-negative patients with unfavourable prognosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlgren J., Stål O., Westman G., Arnesson L. G. Prediction of axillary lymph node metastases in a screened breast cancer population. South-East Sweden Breast Cancer Group. Acta Oncol. 1994;33(6):603–608. doi: 10.3109/02841869409121769. [DOI] [PubMed] [Google Scholar]
- Alam S. M., Whitford P., Cushley W., George W. D., Campbell A. M. Flow cytometric analysis of cell surface carbohydrates in metastatic human breast cancer. Br J Cancer. 1990 Aug;62(2):238–242. doi: 10.1038/bjc.1990.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson C. K., Mouridsen H. T., Zedeler K. Axillary dissection of level I and II lymph nodes is important in breast cancer classification. The Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer. 1992;28A(8-9):1415–1418. doi: 10.1016/0959-8049(92)90534-9. [DOI] [PubMed] [Google Scholar]
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M. S., Locher G. W., Saurer S., Gullick W. J., Waterfield M. D., Groner B., Hynes N. E. Correlation of c-erbB-2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading. Cancer Res. 1988 Mar 1;48(5):1238–1243. [PubMed] [Google Scholar]
- Borg A., Baldetorp B., Fernö M., Killander D., Olsson H., Sigurdsson H. ERBB2 amplification in breast cancer with a high rate of proliferation. Oncogene. 1991 Jan;6(1):137–143. [PubMed] [Google Scholar]
- Bosari S., Lee A. K., DeLellis R. A., Wiley B. D., Heatley G. J., Silverman M. L. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol. 1992 Jul;23(7):755–761. doi: 10.1016/0046-8177(92)90344-3. [DOI] [PubMed] [Google Scholar]
- Brooks S. A., Leathem A. J. Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet. 1991 Jul 13;338(8759):71–74. doi: 10.1016/0140-6736(91)90071-v. [DOI] [PubMed] [Google Scholar]
- Cabanes P. A., Salmon R. J., Vilcoq J. R., Durand J. C., Fourquet A., Gautier C., Asselain B. Value of axillary dissection in addition to lumpectomy and radiotherapy in early breast cancer. The Breast Carcinoma Collaborative Group of the Institut Curie. Lancet. 1992 May 23;339(8804):1245–1248. doi: 10.1016/0140-6736(92)91591-u. [DOI] [PubMed] [Google Scholar]
- Cady B. The need to reexamine axillary lymph node dissection in invasive breast cancer. Cancer. 1994 Feb 1;73(3):505–508. doi: 10.1002/1097-0142(19940201)73:3<505::aid-cncr2820730302>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Chadha M., Chabon A. B., Friedmann P., Vikram B. Predictors of axillary lymph node metastases in patients with T1 breast cancer. A multivariate analysis. Cancer. 1994 Jan 15;73(2):350–353. doi: 10.1002/1097-0142(19940115)73:2<350::aid-cncr2820730219>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Clark G. M., Hilsenbeck S. G., Ravdin P. M., De Laurentiis M., Osborne C. K. Prognostic factors: rationale and methods of analysis and integration. Breast Cancer Res Treat. 1994;32(1):105–112. doi: 10.1007/BF00666211. [DOI] [PubMed] [Google Scholar]
- De Laurentiis M., Ravdin P. M. A technique for using neural network analysis to perform survival analysis of censored data. Cancer Lett. 1994 Mar 15;77(2-3):127–138. doi: 10.1016/0304-3835(94)90095-7. [DOI] [PubMed] [Google Scholar]
- De Laurentiis M., Ravdin P. M. Survival analysis of censored data: neural network analysis detection of complex interactions between variables. Breast Cancer Res Treat. 1994;32(1):113–118. doi: 10.1007/BF00666212. [DOI] [PubMed] [Google Scholar]
- Deckers P. J. Axillary dissection in breast cancer: when, why, how much, and for how long? Another operation soon to be extinct? J Surg Oncol. 1991 Dec;48(4):217–219. doi: 10.1002/jso.2930480402. [DOI] [PubMed] [Google Scholar]
- Fentiman I. S. Axillary surgery in breast cancer: what debate? Eur J Cancer. 1993;29A(6):923–923. doi: 10.1016/s0959-8049(05)80440-x. [DOI] [PubMed] [Google Scholar]
- Fentiman I. S., Chetty U. Axillary surgery in breast cancer--is there still a debate? Eur J Cancer. 1992;28A(6-7):1013–1014. doi: 10.1016/0959-8049(92)90444-7. [DOI] [PubMed] [Google Scholar]
- Fentiman I. S., Mansel R. E. The axilla: not a no-go zone. Lancet. 1991 Jan 26;337(8735):221–223. doi: 10.1016/0140-6736(91)92172-x. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Fisher E. R., Bauer M., Wolmark N., Wickerham D. L., Deutsch M., Montague E., Margolese R., Foster R. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985 Mar 14;312(11):674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- Fisher B. The evolution of paradigms for the management of breast cancer: a personal perspective. Cancer Res. 1992 May 1;52(9):2371–2383. [PubMed] [Google Scholar]
- Greenacre M. Correspondence analysis in medical research. Stat Methods Med Res. 1992;1(1):97–117. doi: 10.1177/096228029200100106. [DOI] [PubMed] [Google Scholar]
- Hennessy C., Henry J. A., May F. E., Westley B. R., Angus B., Lennard T. W. Expression of the antimetastatic gene nm23 in human breast cancer: an association with good prognosis. J Natl Cancer Inst. 1991 Feb 20;83(4):281–285. doi: 10.1093/jnci/83.4.281. [DOI] [PubMed] [Google Scholar]
- Hladiuk M., Huchcroft S., Temple W., Schnurr B. E. Arm function after axillary dissection for breast cancer: a pilot study to provide parameter estimates. J Surg Oncol. 1992 May;50(1):47–52. doi: 10.1002/jso.2930500114. [DOI] [PubMed] [Google Scholar]
- Horak E. R., Leek R., Klenk N., LeJeune S., Smith K., Stuart N., Greenall M., Stepniewska K., Harris A. L. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992 Nov 7;340(8828):1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- Kiricuta C. I., Tausch J. A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer. 1992 May 15;69(10):2496–2501. doi: 10.1002/1097-0142(19920515)69:10<2496::aid-cncr2820691018>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kissin M. W., Querci della Rovere G., Easton D., Westbury G. Risk of lymphoedema following the treatment of breast cancer. Br J Surg. 1986 Jul;73(7):580–584. doi: 10.1002/bjs.1800730723. [DOI] [PubMed] [Google Scholar]
- Lemeshow S., Hosmer D. W., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982 Jan;115(1):92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- Lin P. P., Allison D. C., Wainstock J., Miller K. D., Dooley W. C., Friedman N., Baker R. R. Impact of axillary lymph node dissection on the therapy of breast cancer patients. J Clin Oncol. 1993 Aug;11(8):1536–1544. doi: 10.1200/JCO.1993.11.8.1536. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Margolese R. G. Axillary surgery in breast cancer--there still is a debate. Eur J Cancer. 1993;29A(6):801–801. doi: 10.1016/s0959-8049(05)80411-3. [DOI] [PubMed] [Google Scholar]
- Mathiesen O., Carl J., Bonderup O., Panduro J. Axillary sampling and the risk of erroneous staging of breast cancer. An analysis of 960 consecutive patients. Acta Oncol. 1990;29(6):721–725. doi: 10.3109/02841869009092990. [DOI] [PubMed] [Google Scholar]
- Mazeron J. J., Otmezguine Y., Huart J., Pierquin B. Conservative treatment of breast cancer: results of management of axillary lymph node area in 3353 patients. Lancet. 1985 Jun 15;1(8442):1387–1387. doi: 10.1016/s0140-6736(85)91807-0. [DOI] [PubMed] [Google Scholar]
- Ménard S., Bufalino R., Rilke F., Cascinelli N., Veronesi U., Colnaghi M. I. Prognosis based on primary breast carcinoma instead of pathological nodal status. Br J Cancer. 1994 Oct;70(4):709–712. doi: 10.1038/bjc.1994.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P. M., Clark G. M. A practical application of neural network analysis for predicting outcome of individual breast cancer patients. Breast Cancer Res Treat. 1992;22(3):285–293. doi: 10.1007/BF01840841. [DOI] [PubMed] [Google Scholar]
- Ravdin P. M., Clark G. M., Hilsenbeck S. G., Owens M. A., Vendely P., Pandian M. R., McGuire W. L. A demonstration that breast cancer recurrence can be predicted by neural network analysis. Breast Cancer Res Treat. 1992;21(1):47–53. doi: 10.1007/BF01811963. [DOI] [PubMed] [Google Scholar]
- Ravdin P. M., Clark G. M., Hough J. J., Owens M. A., McGuire W. L. Neural Network Analysis of DNA flow cytometry histograms. Cytometry. 1993;14(1):74–80. doi: 10.1002/cyto.990140113. [DOI] [PubMed] [Google Scholar]
- Ravdin P. M., De Laurentiis M., Vendely T., Clark G. M. Prediction of axillary lymph node status in breast cancer patients by use of prognostic indicators. J Natl Cancer Inst. 1994 Dec 7;86(23):1771–1775. doi: 10.1093/jnci/86.23.1771. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Senofsky G. M., Ketcham A. S. Role and extent of lymphadenectomy for early breast cancer. Semin Surg Oncol. 1992 Mar-Apr;8(2):78–82. doi: 10.1002/ssu.2980080206. [DOI] [PubMed] [Google Scholar]
- Rose C. M., Botnick L. E., Weinstein M., Harris J. R., Koufman C., Silen W., Hellman S. Axillary sampling in the definitive treatment of breast cancer by radiation therapy and lumpectomy. Int J Radiat Oncol Biol Phys. 1983 Mar;9(3):339–344. doi: 10.1016/0360-3016(83)90293-6. [DOI] [PubMed] [Google Scholar]
- Royds J. A., Stephenson T. J., Rees R. C., Shorthouse A. J., Silcocks P. B. Nm23 protein expression in ductal in situ and invasive human breast carcinoma. J Natl Cancer Inst. 1993 May 5;85(9):727–731. doi: 10.1093/jnci/85.9.727. [DOI] [PubMed] [Google Scholar]
- Shek L. L., Godolphin W. Model for breast cancer survival: relative prognostic roles of axillary nodal status, TNM stage, estrogen receptor concentration, and tumor necrosis. Cancer Res. 1988 Oct 1;48(19):5565–5569. [PubMed] [Google Scholar]
- Tabár L., Fagerberg C. J., Gad A., Baldetorp L., Holmberg L. H., Gröntoft O., Ljungquist U., Lundström B., Månson J. C., Eklund G. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985 Apr 13;1(8433):829–832. doi: 10.1016/s0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- Tabár L., Fagerberg G., Day N. E., Duffy S. W., Kitchin R. M. Breast cancer treatment and natural history: new insights from results of screening. Lancet. 1992 Feb 15;339(8790):412–414. doi: 10.1016/0140-6736(92)90090-p. [DOI] [PubMed] [Google Scholar]
- Tjandra J. J., Russell I. S., Collins J. P., Andrews J. T., Lichtenstein M., Binns D., McKenzie I. F. Immunolymphoscintigraphy for the detection of lymph node metastases from breast cancer. Cancer Res. 1989 Mar 15;49(6):1600–1608. [PubMed] [Google Scholar]
- Todd J. H., Dowle C., Williams M. R., Elston C. W., Ellis I. O., Hinton C. P., Blamey R. W., Haybittle J. L. Confirmation of a prognostic index in primary breast cancer. Br J Cancer. 1987 Oct;56(4):489–492. doi: 10.1038/bjc.1987.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma S., Leonessa F., Romanini A., Badellino F., Bonassi S., Nicolo G., Rosso R. Predictive value of some clinical and pathological parameters on upper level axillary lymph node involvement in breast cancer. Anticancer Res. 1991 Jul-Aug;11(4):1439–1443. [PubMed] [Google Scholar]
- Weidner N., Folkman J., Pozza F., Bevilacqua P., Allred E. N., Moore D. H., Meli S., Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992 Dec 16;84(24):1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- van Dongen J. A. Subclavicular biopsy as a guideline for the treatment of breast cancer. World J Surg. 1977 May;1(3):306–308. doi: 10.1007/BF01556844. [DOI] [PubMed] [Google Scholar]


