Abstract
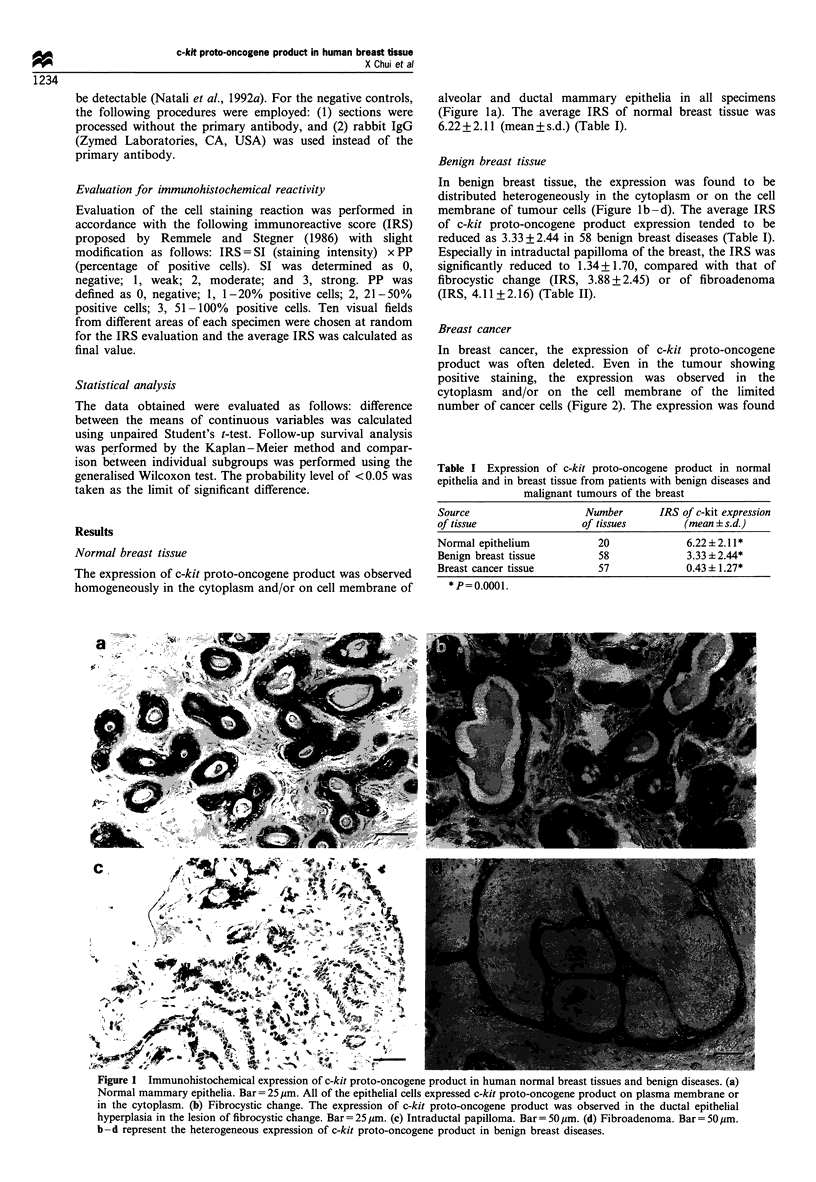
The immunohistochemical expression of c-kit proto-oncogene product in 57 breast cancer tissues was studied using anti-c-kit proto-oncogene product antibody in comparison with 20 normal breast tissues and 58 benign breast tumours. In normal breast tissues, the c-kit proto-oncogene product was strongly expressed on cell membrane and/or cytoplasm of alveolar and ductal cells. The immunoreactive score (IRS) of c-kit proto-oncogene product in normal mammary epithelia was 6.22 +/- 2.11 (mean +/- s.d.). In benign breast diseases, the c-kit proto-oncogene product was detected heterogeneously with a reduced IRS (3.33 +/- 2.44). In breast cancer tissues, the expression of the immunoreactive c-kit proto-oncogene product was often deleted and the average IRS was significantly reduced compared to those of normal breast tissues or benign breast diseases tissues. Among benign diseases, the average IRS of intraductal papilloma was significantly reduced (1.34 +/- 1.70) and the staining intensity and pattern were found to be similar to those seen in breast cancer. The results in this study suggested that the c-kit proto-oncogene product is correlated with the growth control or the differentiation of normal breast epithelium. Also, the loss of the expression of this protein may indicate the change of the signal transduction in relation to malignant transformation in human mammary epithelium.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flanagan J. G., Chan D. C., Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991 Mar 8;64(5):1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Keshet E., Lyman S. D., Williams D. E., Anderson D. M., Jenkins N. A., Copeland N. G., Parada L. F. Embryonic RNA expression patterns of the c-kit receptor and its cognate ligand suggest multiple functional roles in mouse development. EMBO J. 1991 Sep;10(9):2425–2435. doi: 10.1002/j.1460-2075.1991.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda R., Takahashi T., Nakamura S., Sekido Y., Nishida K., Seto M., Seito T., Sugiura T., Ariyoshi Y., Takahashi T. Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol. 1993 Jan;142(1):339–346. [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Sures I., Mottolese M., Botti C., Ullrich A. Breast cancer is associated with loss of the c-kit oncogene product. Int J Cancer. 1992 Nov 11;52(5):713–717. doi: 10.1002/ijc.2910520508. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Sures I., Santoro E., Bigotti A., Ullrich A. Expression of c-kit receptor in normal and transformed human nonlymphoid tissues. Cancer Res. 1992 Nov 15;52(22):6139–6143. [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Winkler A. B., Cavaliere R., Bigotti A., Ullrich A. Progression of human cutaneous melanoma is associated with loss of expression of c-kit proto-oncogene receptor. Int J Cancer. 1992 Sep 9;52(2):197–201. doi: 10.1002/ijc.2910520207. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Motomura K., Inaji H., Imaoka S., Koyama H. Clonal analysis of human breast cancer by means of the polymerase chain reaction. Cancer Res. 1992 Dec 1;52(23):6594–6597. [PubMed] [Google Scholar]
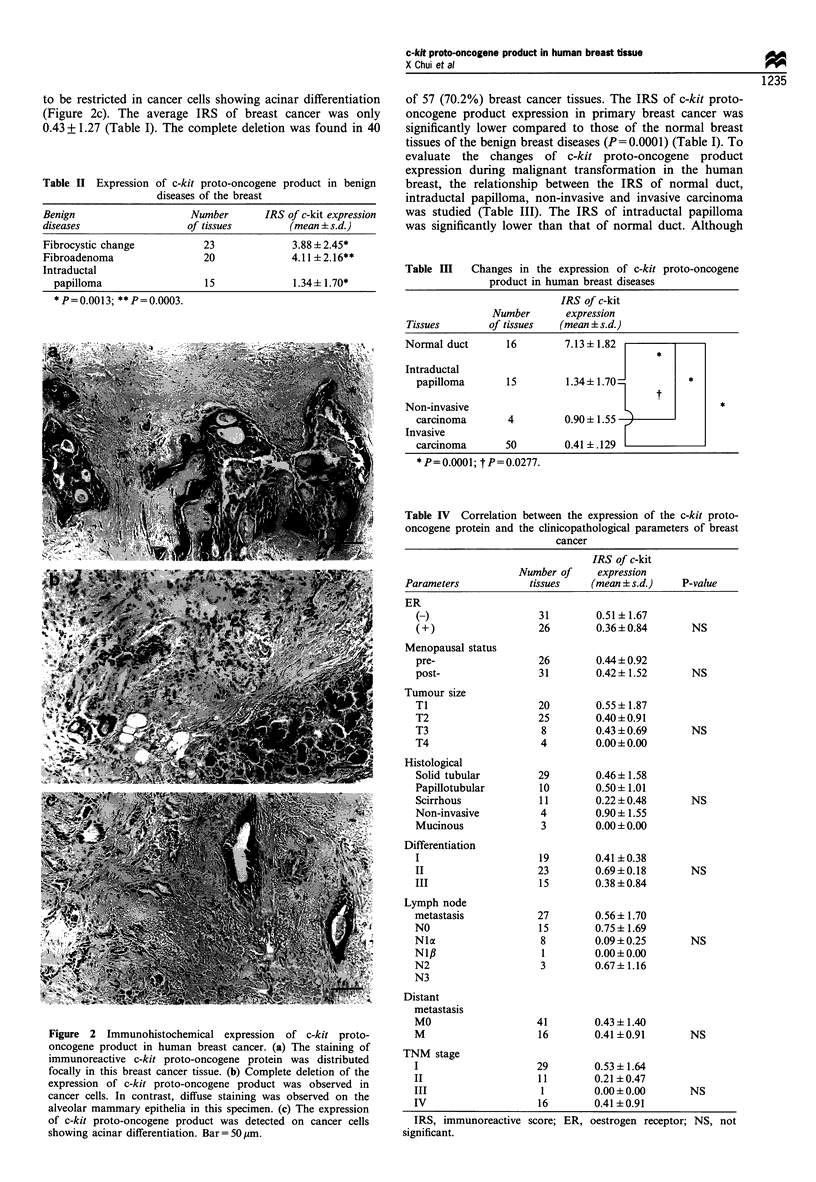
- Noguchi S., Motomura K., Inaji H., Imaoka S., Koyama H. Clonal analysis of predominantly intraductal carcinoma and precancerous lesions of the breast by means of polymerase chain reaction. Cancer Res. 1994 Apr 1;54(7):1849–1853. [PubMed] [Google Scholar]
- Qiu F. H., Ray P., Brown K., Barker P. E., Jhanwar S., Ruddle F. H., Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988 Apr;7(4):1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith A. D., Ellis C., Lyman S. D., Anderson D. M., Williams D. E., Bernstein A., Pawson T. Signal transduction by normal isoforms and W mutant variants of the Kit receptor tyrosine kinase. EMBO J. 1991 Sep;10(9):2451–2459. doi: 10.1002/j.1460-2075.1991.tb07784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeyer T., Peter S., Hartmann M., Munemitsu S., Ackermann R., Ullrich A., Slamon D. J. Expression of the hst-1 and c-kit protooncogenes in human testicular germ cell tumors. Cancer Res. 1991 Apr 1;51(7):1811–1816. [PubMed] [Google Scholar]
- Wershil B. K., Tsai M., Geissler E. N., Zsebo K. M., Galli S. J. The rat c-kit ligand, stem cell factor, induces c-kit receptor-dependent mouse mast cell activation in vivo. Evidence that signaling through the c-kit receptor can induce expression of cellular function. J Exp Med. 1992 Jan 1;175(1):245–255. doi: 10.1084/jem.175.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]