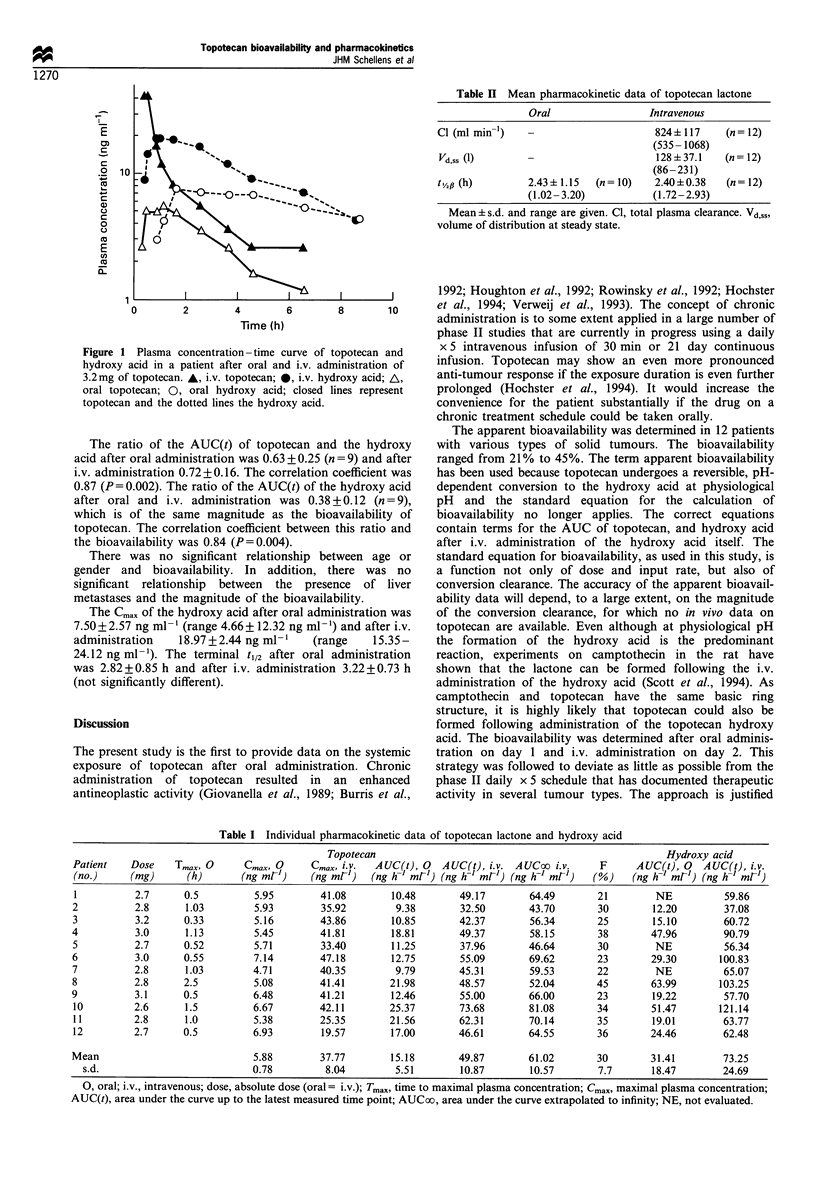
Abstract
The results of preclinical and clinical studies indicate enhanced antineoplastic activity of topotecan (SKF 104864-A) when administered as a chronic treatment. We determined the apparent bioavailability and pharmacokinetics of topotecan administered orally to 12 patients with solid tumours in a two-part crossover study. The oral dose of 1.5 mg m-2 was administered as a drinking solution of 200 ml on day 1. The i.v. dose of 1.5 mg m-2 was administered as a 30 min continuous infusion on day 2. The bioavailability was calculated as the ratio of the oral to i.v. area under the curve (AUC) calculated up to the last measured time point. The oral drinking solution was well tolerated. The bioavailability revealed moderate inter-patient variation and was 30% +/- 7.7% (range 21-45%). The time to maximum plasma concentration after oral administration (Tmax) was 0.78 h (median; range 0.33-2.5). Total i.v. plasma clearance of topotecan was 824 +/- 154 ml min-1 (range 535-1068 ml min(-1)). The AUC ratio of topotecan and the lactone ring-opened hydrolysis product (hydroxy acid) was of the same order after oral (0.34-1.13) and i.v. (0.47-0.98) administration. The bioavailability of topotecan after oral administration illustrates significant systemic exposure to the drug which may enable chronic oral treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beijnen J. H., Smith B. R., Keijer W. J., van Gijn R., ten Bokkel Huinink W. W., Vlasveld L. T., Rodenhuis S., Underberg W. J. High-performance liquid chromatographic analysis of the new antitumour drug SK&F 104864-A (NSC 609699) in plasma. J Pharm Biomed Anal. 1990;8(8-12):789–794. doi: 10.1016/0731-7085(90)80122-6. [DOI] [PubMed] [Google Scholar]
- Blaney S. M., Balis F. M., Cole D. E., Craig C., Reid J. M., Ames M. M., Krailo M., Reaman G., Hammond D., Poplack D. G. Pediatric phase I trial and pharmacokinetic study of topotecan administered as a 24-hour continuous infusion. Cancer Res. 1993 Mar 1;53(5):1032–1036. [PubMed] [Google Scholar]
- Burris H. A., 3rd, Hanauske A. R., Johnson R. K., Marshall M. H., Kuhn J. G., Hilsenbeck S. G., Von Hoff D. D. Activity of topotecan, a new topoisomerase I inhibitor, against human tumor colony-forming units in vitro. J Natl Cancer Inst. 1992 Dec 2;84(23):1816–1820. doi: 10.1093/jnci/84.23.1816. [DOI] [PubMed] [Google Scholar]
- Creemers G. J., Lund B., Verweij J. Topoisomerase I inhibitors: topotecan and irenotecan. Cancer Treat Rev. 1994 Jan;20(1):73–96. doi: 10.1016/0305-7372(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S., Wall M. E., Wani M. C., Nicholas A. W., Liu L. F., Silber R., Potmesil M. DNA topoisomerase I--targeted chemotherapy of human colon cancer in xenografts. Science. 1989 Nov 24;246(4933):1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- Hochster H., Liebes L., Speyer J., Sorich J., Taubes B., Oratz R., Wernz J., Chachoua A., Raphael B., Vinci R. Z. Phase I trial of low-dose continuous topotecan infusion in patients with cancer: an active and well-tolerated regimen. J Clin Oncol. 1994 Mar;12(3):553–559. doi: 10.1200/JCO.1994.12.3.553. [DOI] [PubMed] [Google Scholar]
- Houghton P. J., Cheshire P. J., Myers L., Stewart C. F., Synold T. W., Houghton J. A. Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol. 1992;31(3):229–239. doi: 10.1007/BF00685553. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988 Apr 1;48(7):1722–1726. [PubMed] [Google Scholar]
- Kingsbury W. D., Boehm J. C., Jakas D. R., Holden K. G., Hecht S. M., Gallagher G., Caranfa M. J., McCabe F. L., Faucette L. F., Johnson R. K. Synthesis of water-soluble (aminoalkyl)camptothecin analogues: inhibition of topoisomerase I and antitumor activity. J Med Chem. 1991 Jan;34(1):98–107. doi: 10.1021/jm00105a017. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Grochow L. B., Hendricks C. B., Ettinger D. S., Forastiere A. A., Hurowitz L. A., McGuire W. P., Sartorius S. E., Lubejko B. G., Kaufmann S. H. Phase I and pharmacologic study of topotecan: a novel topoisomerase I inhibitor. J Clin Oncol. 1992 Apr;10(4):647–656. doi: 10.1200/JCO.1992.10.4.647. [DOI] [PubMed] [Google Scholar]
- Scott D. O., Bindra D. S., Stella V. J. Plasma pharmacokinetics of lactone and carboxylate forms of 20(S)-camptothecin in anesthetized rats. Pharm Res. 1993 Oct;10(10):1451–1457. doi: 10.1023/a:1018919224450. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Beal S. L. Pharmacokinetic parameter estimates from several least squares procedures: superiority of extended least squares. J Pharmacokinet Biopharm. 1985 Apr;13(2):185–201. doi: 10.1007/BF01059398. [DOI] [PubMed] [Google Scholar]
- Verweij J., Lund B., Beijnen J., Planting A., de Boer-Dennert M., Koier I., Rosing H., Hansen H. Phase I and pharmacokinetics study of topotecan, a new topoisomerase I inhibitor. Ann Oncol. 1993 Sep;4(8):673–678. doi: 10.1093/oxfordjournals.annonc.a058623. [DOI] [PubMed] [Google Scholar]
- Wall J. G., Burris H. A., 3rd, Von Hoff D. D., Rodriguez G., Kneuper-Hall R., Shaffer D., O'Rourke T., Brown T., Weiss G., Clark G. A phase I clinical and pharmacokinetic study of the topoisomerase I inhibitor topotecan (SK&F 104864) given as an intravenous bolus every 21 days. Anticancer Drugs. 1992 Aug;3(4):337–345. doi: 10.1097/00001813-199208000-00004. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]



